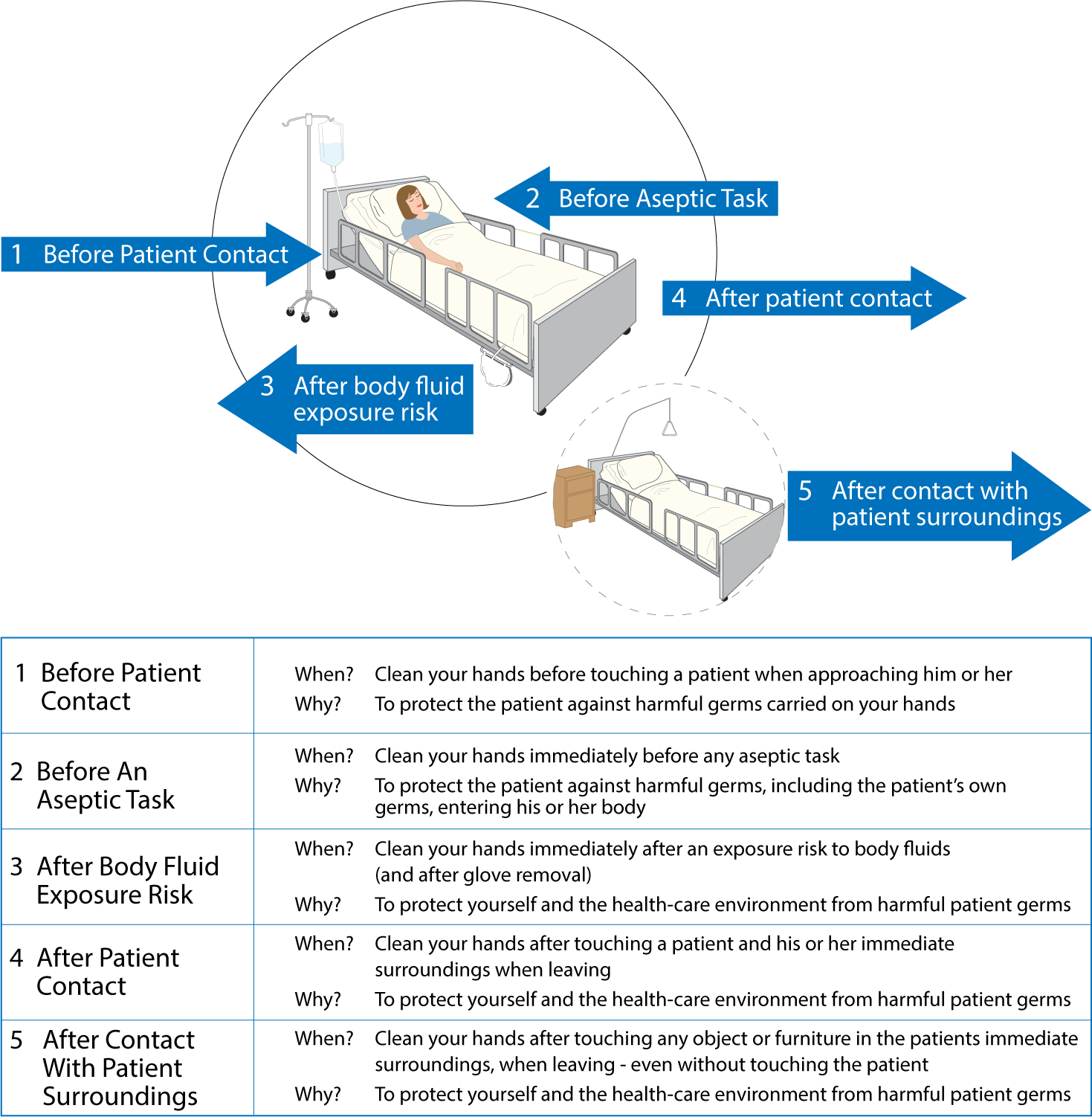
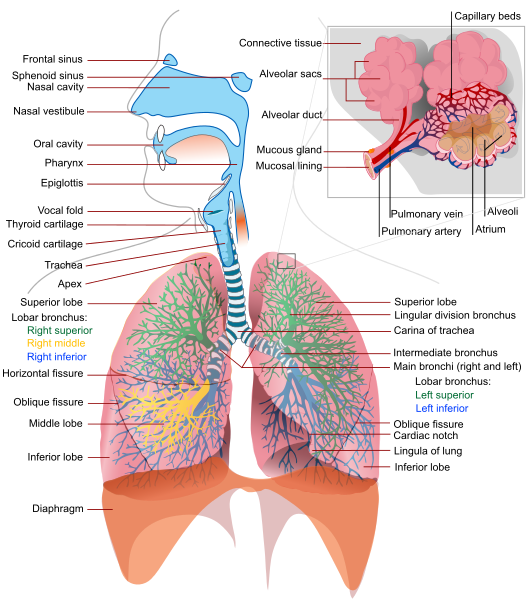
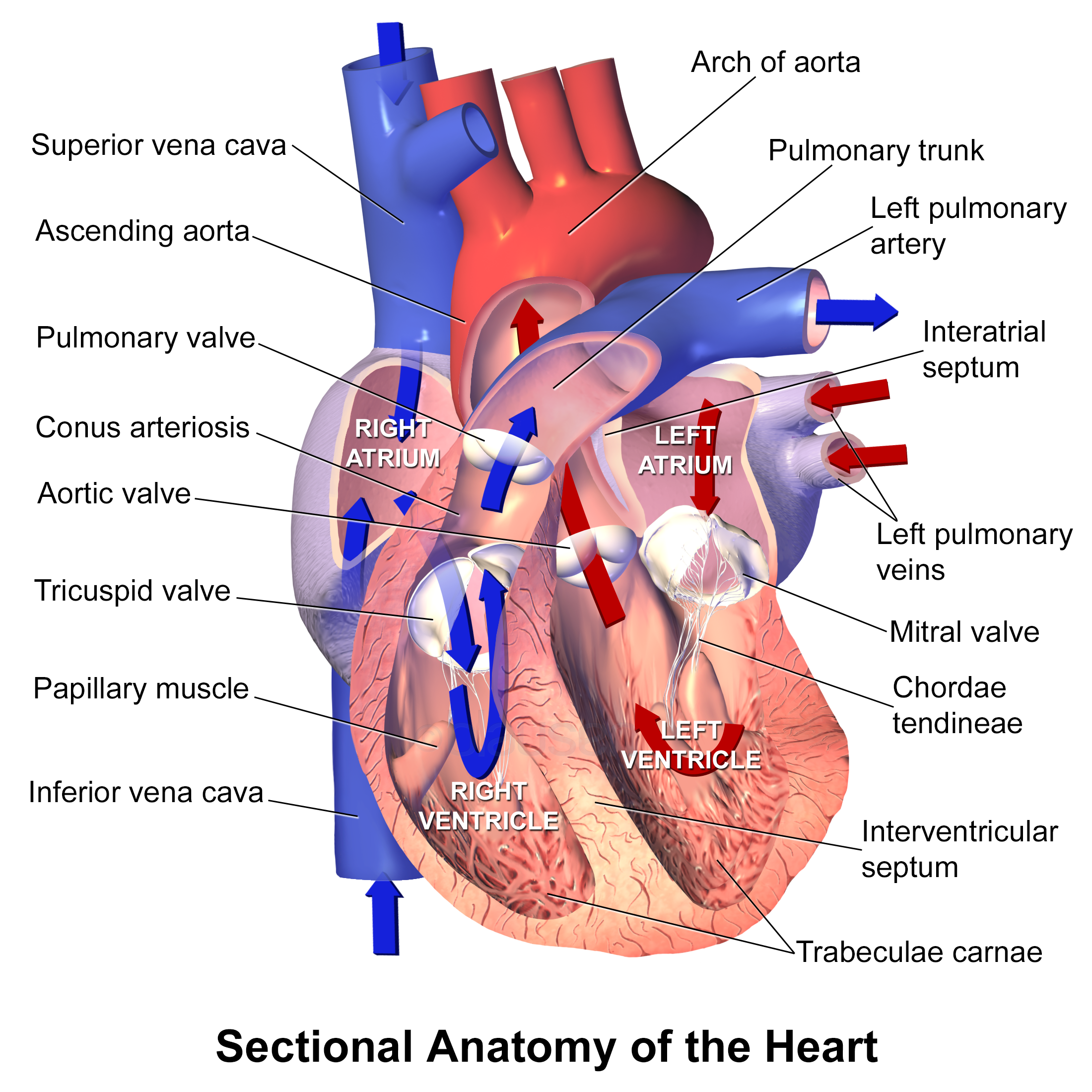
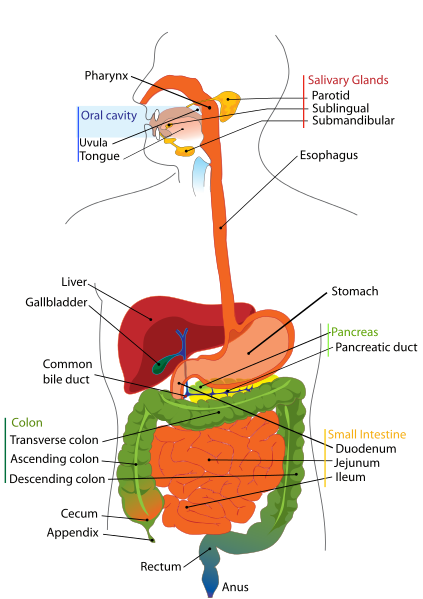
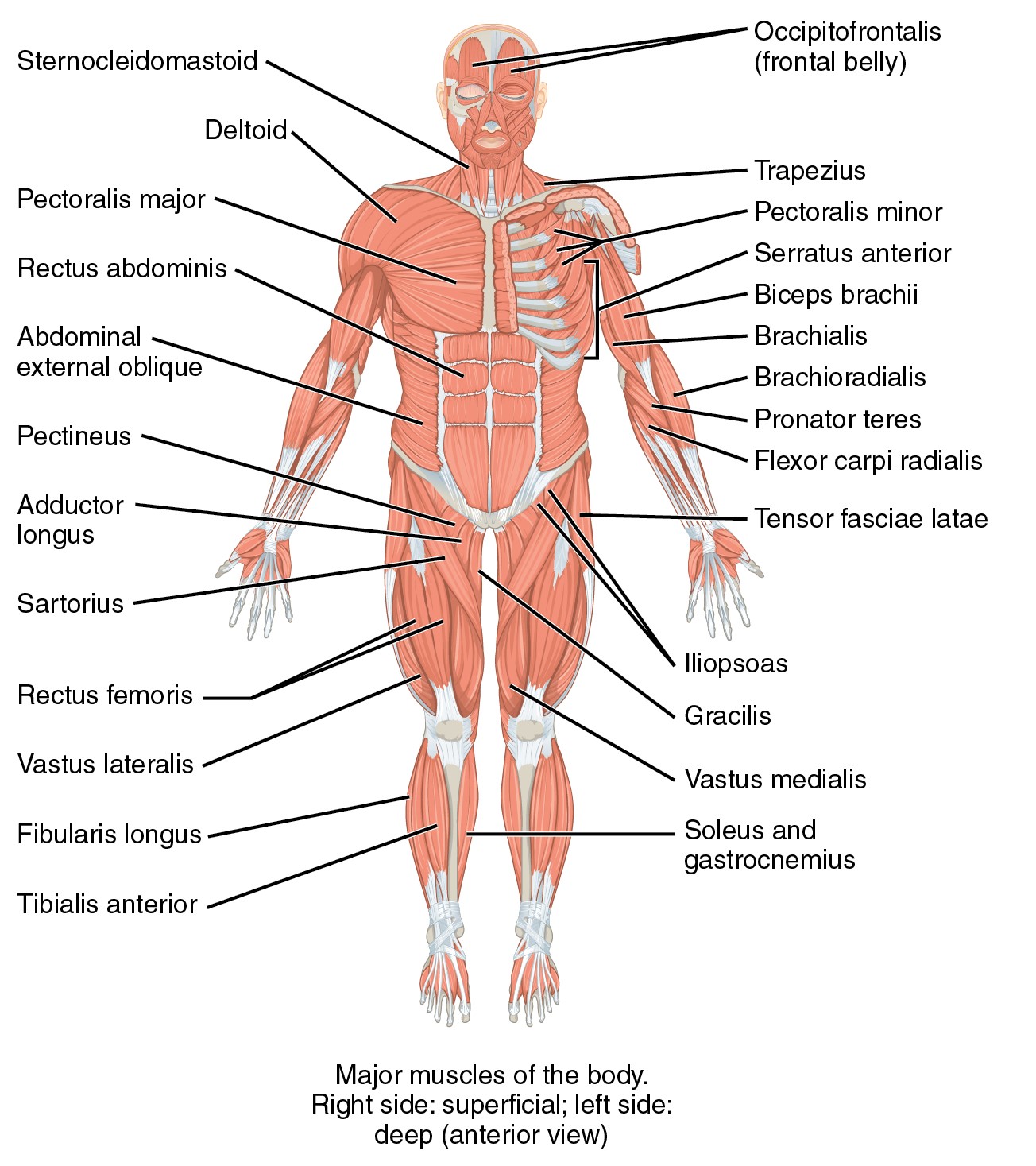
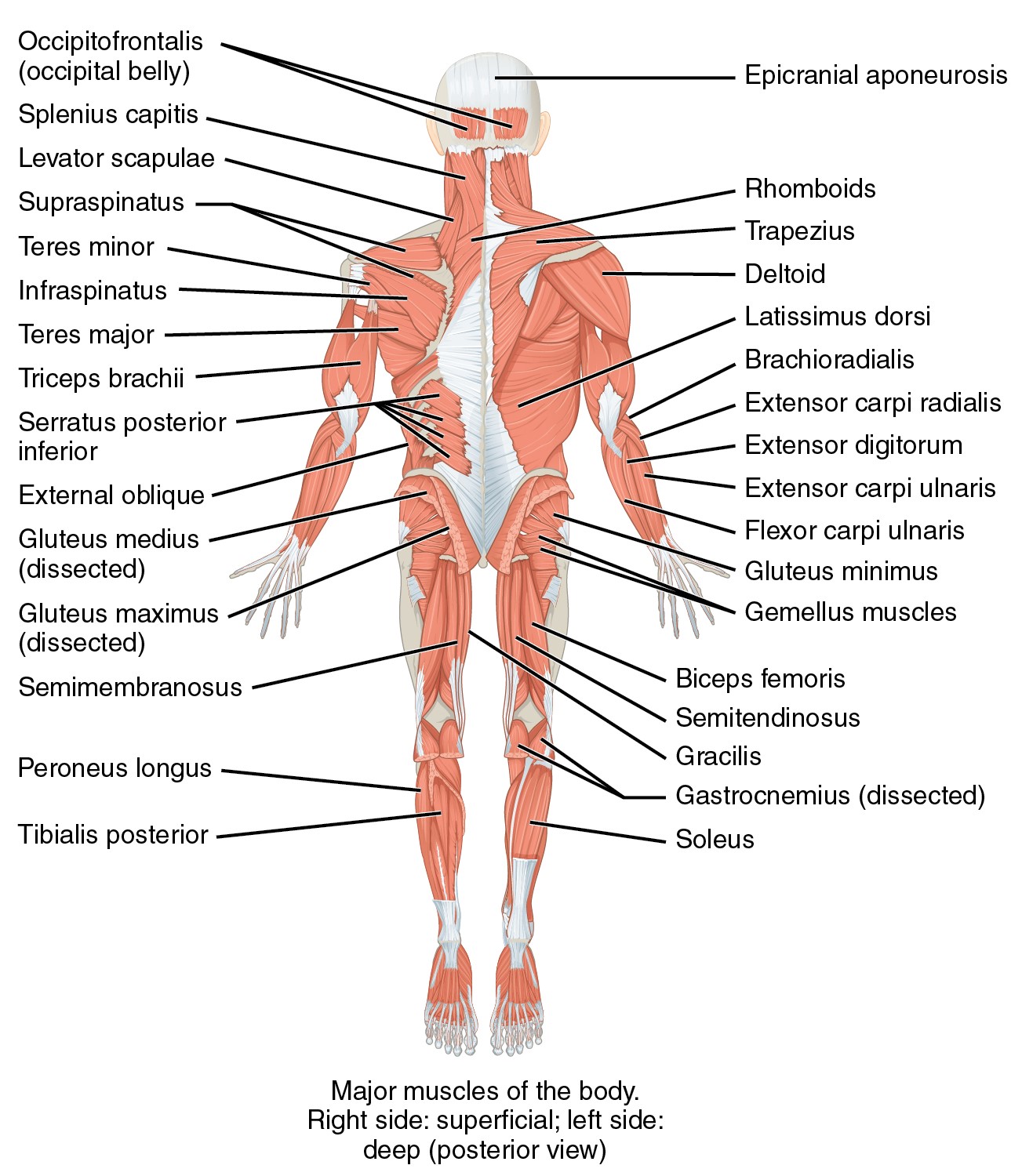
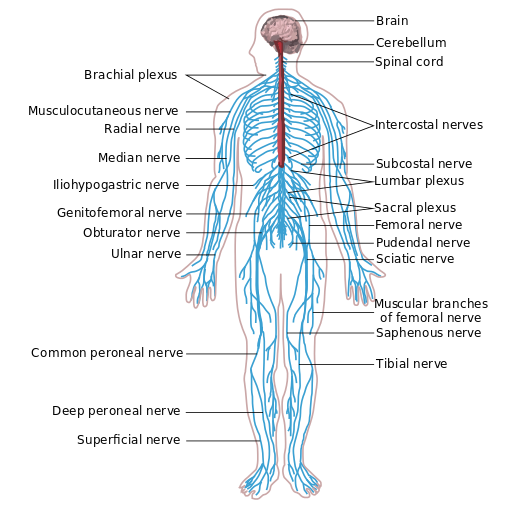
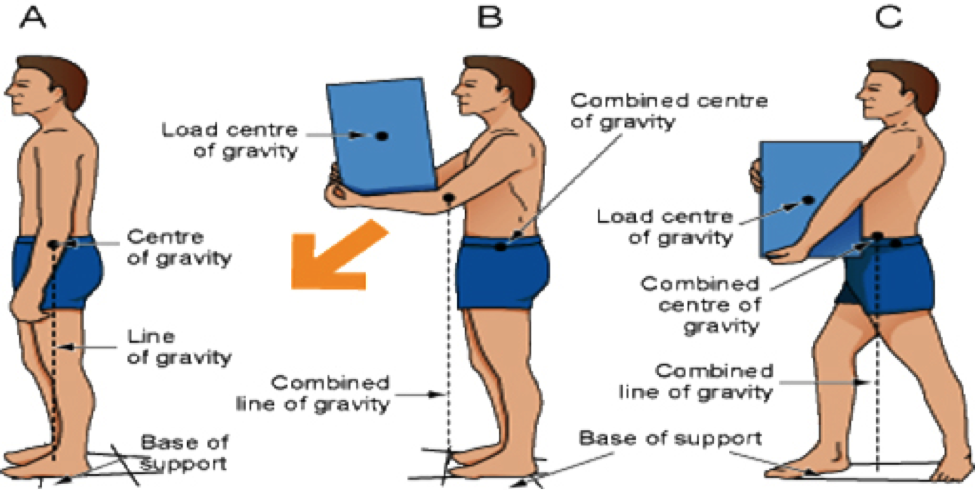
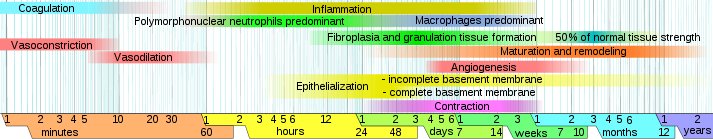
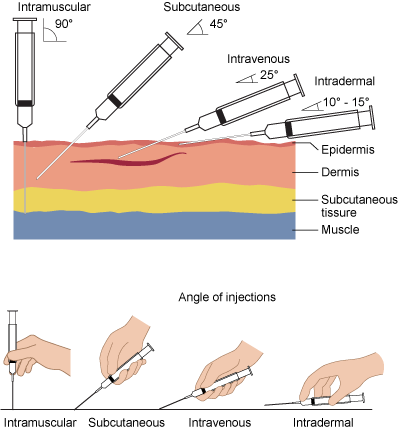
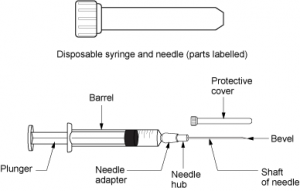
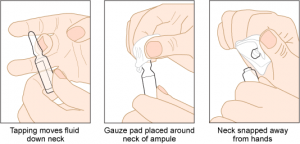
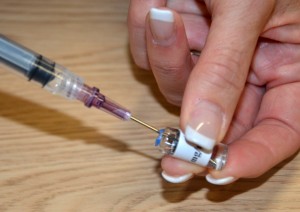
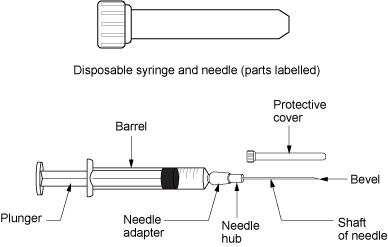
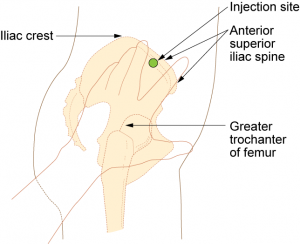
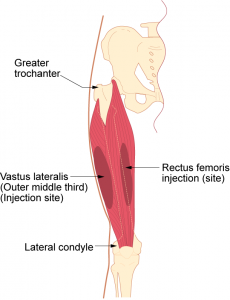
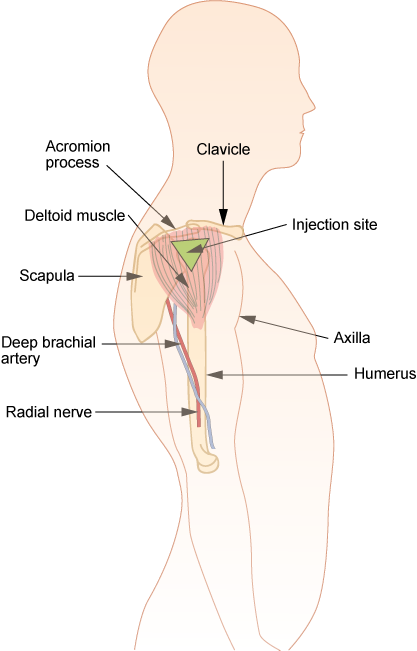
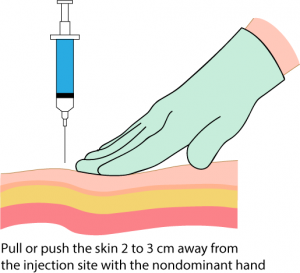
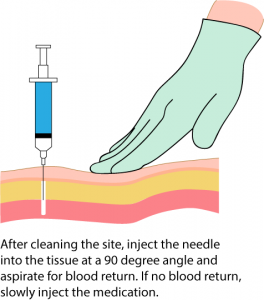
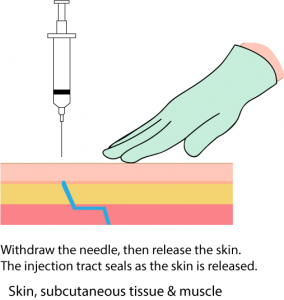
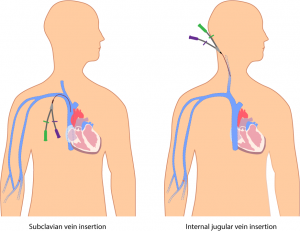
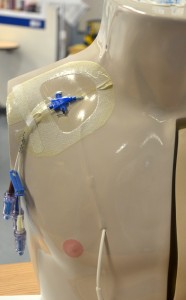
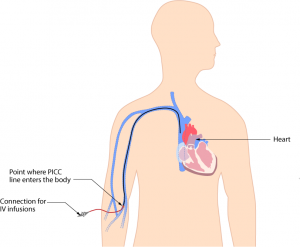
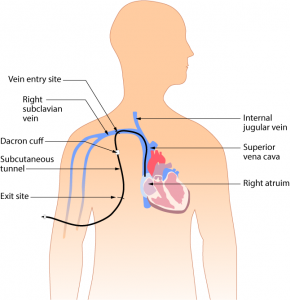
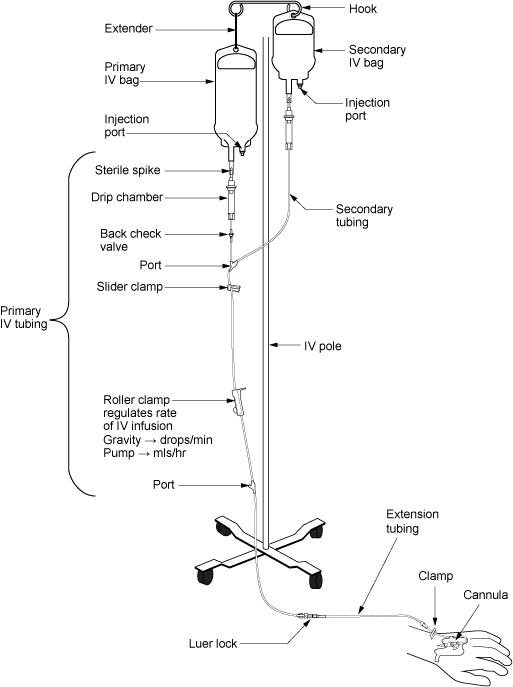
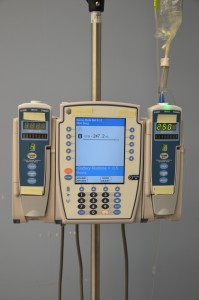
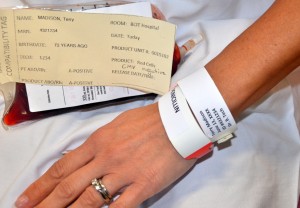
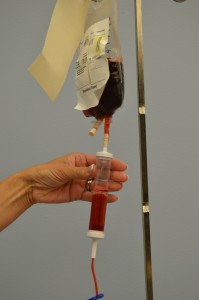
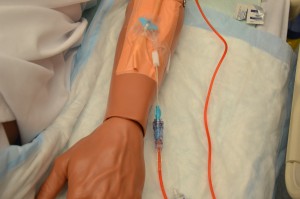
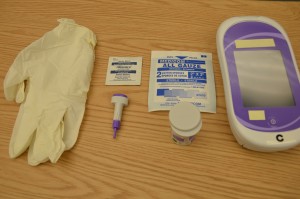
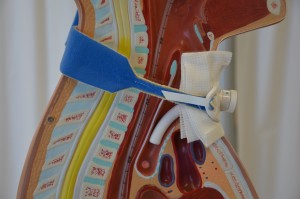
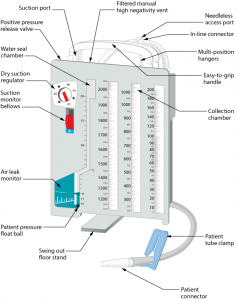
Parenteral medication administration is an effective method of delivering medication to patients, and can be safely accomplished by utilizing the appropriate guidelines and policies in place to keep patients safe from harm. IV medications have a higher risk of harm than non-parenteral medication. The ever-increasing complexity of the health care environment increases the risk of a medication error with parenteral medications. The key takeaways below provide advice for preventing errors with parenteral medications.
- Parenteral medications have a quicker onset of therapeutic effects. Be aware of the onset, peak, and duration of all parenteral medications.
- Know which medications are considered high-alert medications and perform independent double checks to minimize errors.
- Always consider the therapeutic effects and adverse effects when administering parenteral medications.
- Safeguards for medication administration exist in most hospitals. Make use of all safety strategies (such as smart IV pumps, no-interruption zones, two patient identifiers, and checklists) to administer medications safely.
- Quality and safety controls for safe medication administration must be considered along the entire process, not just at the point of administration.
- It is human nature to look for quick and easy ways to perform a task, but doing so may lead to errors. Avoid workarounds: Most hospitals have operational failures that lead to front line health care providers finding ways to manage deficiency in hospital operating systems. Rather than creating workarounds, engage in the additional steps to prevent re-occurrence of issues.
- Stay current with evidence-based research regarding potential system errors in health care. Commit to improving patient safety with medication administration.
- Report all errors, near misses, and adverse reactions to ensure knowledge is shared, and to prevent further errors from occurring.
Suggested Online Resources
- Agency for Healthcare Research and Quality (AHRQ). This website provides evidence-based research, guidelines, recommendations, and resources on improving patient safety .
- Canadian Patient Safety Institute (CPSI). This organization’s website provides guidelines, research, and recommendations for improving patient safety in Canada.
- Drug calculations. This medication calculation website reviews how to calculate the dosages for parenteral and non-parenteral medications, and IV fluids. It also includes metric conversions and IV drop rate calculations.
- Institute for Healthcare Improvement (IHI). This group’s website provides educational resources, webinars, publications, and improvement stories and tools to enhance patient safety.
- Institute for Safe Medication Practices (ISMP). This organization focuses on improving medication administration. Its website lists high-alert medications, offers newsletters and webinars, and provides a system for reporting medication errors, guidelines, and policies on safe practices.
References
Ağac, E., & Günes, U. Y. (2011). Effect on pain of changing the needle prior to administering medication intramuscularly: A randomized controlled trial. Journal of Advanced Nursing, 67(3) 563-568. doi: 10.1111/j.1365-2648.2010.05513.x.
Agency for Healthcare Research and Quality. (2014). Checklists. Retrieved on June 11, 2015, from http://psnet.ahrq.gov/primer.aspx?primerID=14
Alberta Health Services. (2009). Direct IV medications. Specialized clinical competency program. Retrieved on June 2, 2015, from http://extcontent.covenanthealth.ca/Policies/iii-45_Learning_Module.pdf
American Nurses Association. (2002). Needlestick prevention guide. Retrieved on Oct 5, 2015, from http://www.who.int/occupational_health/activities/2needguid.pdf
ATI. (2015a). Administering an ID injection. Retrieved on Oct 7, 2015, from http://www.atitesting.com/ati_next_gen/skillsmodules/content/medication-administration-3/equipment/intradermal-injections.html
ATI. (2015b). Administering an SC injection. Retrieved on Oct 7, 2015, from http://www.atitesting.com/ati_next_gen/skillsmodules/content/medication-administration-3/viewing/subcutaneous_injection.html
Berman, A., & Snyder S. J. (2016). Skills in clinical nursing (8th ed.). New Jersey, New York: Pearson.
Black, L. (2013). Ditch the pinch: Bilateral exposure injuries during subcutaneous injections. American Journal of Infection Control, 41(9), 815-819. doi: 10.1016/j.ajic.2012.10.017
British Columbia Institute of Technology (BCIT). (2015). NURS 3020: Clinical techniques. Week 11, power point notes.
Brookside Associates. (2015a). Procedure for administering an intradermal injection. Retrieved on Oct 8, 2015, from http://brooksidepress.org/intramuscular/lessons/lesson-2-administer-an-intramuscular-injection/2-07-procedure-for-administering-an-intradermal-injection/
Brookside Associates. (2015b). Procedure for administering a subcutaneous injection. Retrieved on Oct 7, 2015, from http://brooksidepress.org/intramuscular/lessons/lesson-2-administer-an-intramuscular-injection/2-05-procedure-for-administering-a-subcutaneous-injection/
Butt, A. R. (2010). Medical error in Canada. Issues related to reporting medical error and methods to increase reporting. McMaster University Medical Journal, Clincal Review, 7(1). 15-18.
Canadian Agency for Drugs and Technologies in Health. (2014). Aspirating versus not aspirating prior to injection of medication: Comparative clinical evidence and guidelines. Retrieved on Oct 7, 2015, from
https://www.cadth.ca/sites/default/files/pdf/htis/nov-2014/RB0669%20Aspiration%20Prior%20to%20Injection%20Final.pdf
Canadian Diabetes Association. Clinical Practice Guidelines Expert Committee. (2013). Clinical practice guidelines for the prevention and management of diabetes in Canada. Canadian journal of diabetes, 37(supp 1), s1-s212.
Canadian Institute for Health Information. (2009). National system for incident reporting: An innovative approach to information management. CPSI forum, 2009. Retrieved on June 2, 2015, from http://www.patientsafetyinstitute.ca/English/toolsResources/Presentations/Documents/2009/Canada%27s%20Forum%20-%20Presentation%20A4-b.pdf
Canadian Patient Safety Institute. (2012). Canadian analysis incident framework. Retrieved on June 3, 2015, from http://www.patientsafetyinstitute.ca/english/toolsresources/incidentanalysis/documents/canadian%20incident%20analysis%20framework.pdf
Centers for Disease Control (CDC). (2012). NOISH fast facts (home healthcare workers). How to prevent needlestick and sharp injuries. Retrieved on Oct 7, 2015, from http://www.cdc.gov/niosh/docs/2012-123/pdfs/2012-123.pdf
Centers for Disease Control (CDC). (2013). Infection control. Retrieved on June 11, 2015, from http://www.cdc.gov/oralhealth/infectioncontrol/faq/protective_equipment.htm
Centers for Disease Control (CDC). (2015). Injection safety. Retrieved on June 2, 2015, from http://www.cdc.gov/injectionsafety/
College of Nurses of Ontario. (2015). Medication practice standard. Retrieved on June 1, 2015, from http://www.cno.org/Global/docs/prac/41007_Medication.pdf
Clayton, B. D., Stock, Y. N., & Cooper, S. E. (2010). Basic pharmacology for nurses. St Louis, MO: Mosby Elsevier.
Cocoman, A., & Murray, J. (2008). Intramuscular injections: A review of best practice for mental health nurses. Journal of Psychiatry Mental Health Nursing, 15(5), 424-434. doi: 10.1111/j.1365-2850.2007.01236
Davidson, K.M. & Rourke, L. (2014). Teaching best evidence: Deltoid intramuscular injection techniques. Retrieved on Oct 15, 2015, from http://www.sciedu.ca/journal/index.php/jnep/article/view/1888/1291
Debono, D. S., Greenfield, D., Travaglia, J. F., Long, J. C., Black, D., Johnson, J., & Braithwaite, J. (2013). Nurses’ workarounds in acute healthcare settings: A scoping review. BMC Health Services Research, 13(175). doi:10.1186/1472-6963-13-175
Ellis, J., & Parush, A. (2012). Preventing errors with high alert drugs: Testing a computerized double check. Retrieved on June 5, 2015, from http://www.patientsafetyinstitute.ca/English/research/cpsiResearchCompetitions/2009/Documents/Ellis/Ellis%20Techinical%20Report%20English.pdf
Greenway, K. (2014). Rituals in nursing: Intramuscular injection. Journal of Clinical Nursing, 23(23-24), 3583-3588. doi: 10.1111/jocn.12627
Health Canada. (2012). Adverse reaction information. Retrieved on June 1, 2015, from http://www.hc-sc.gc.ca/dhp-mps/medeff/advers-react-neg/index-eng.php
Hunter, J. (2008). Intramuscular injection techniques. Nursing Standard, 22 (24), 35-40.
Hutin, Y., Hauri, A., Chiarello, L., Caitlin, M., Stillwell, B., Ghebrehiwet, T., Garner, J., and Members of the Safe Injection Practice Group (2003). Best infection control practices for intradermal, subcutaneous and intramuscular injections. Retrieved on Nov. 8, 2015 from http://www.who.int/bulletin/volumes/81/7/Hutin0703.pdf
Institute for Healthcare Improvement. (2015). High alert medication safety. Retrieved on June 11, 2015, from http://www.ihi.org/topics/highalertmedicationsafety/pages/default.aspx
Institute for Safe Medication Practices (ISMP). (2003). How fast is too fast for IV push medications? Retrieved on June 3, 2015, from http://www.ismp.org/newsletters/acutecare/articles/20030515.asp
Institute for Safe Medication Practices (ISMP). (2013). Independent double checks: Undervalued and misused: Selective use of this strategy can play an important role in medication safety. Retrieved on June 5, 2015, from https://www.ismp.org/newsletters/acutecare/showarticle.aspx?id=51
Institute for Safe Medication Practices (ISMP). (2014). ISMP list of high alert medications in acute care settings. Retrieved on June 1, 2015, from https://www.ismp.org/tools/highalertmedications.pdf
Institute for Safe Medication Practices Canada (ISMP Canada). (2013). Ontario hospital critical incident related to medications or IV fluids analysis report. January-December 2013. Retrieved on June 4, 2015, from http://www.ismp-canada.org/download/ocil/ON_Critical_Incidents_Analysis_Report_3JUL2014.pdf
Institute for Safe Medication Practices Canada (ISMP Canada). (2014). Opioid-related deaths associated with medication incidents. Findings of multi-incident analysis. Retrieved on June 4, 2015, from http://www.ismp-canada.org/download/OpioidStewardship/Opioid-Related_Deaths_Associated_with_Medication_Incidents_Nov2014.pdf
Ipp, M., Sam, J., & Parkin, P. C. (2006). Needle aspiration and intramuscular vaccination. Arch Pediatr Adolesc Med, 160(4), 451-453. doi:10.1001/archpedi.160.4.451-a
Lynn, P. (2011). Photo atlas of medication administration (4th ed.). Philadelphia, PA: Lippincott Williams & Wilkins.
Malkin, B. (2008). Are techniques used for intramuscular injection based on research evidence? Nursing times, 104: 50-51, 48–51.
Merriam-Webster. (2015). Definition of parenteral. Retrieved on June 5, 2015, from http://www.merriam-webster.com/medical/parenteral
Mitchell, J. R., & Whitney, F. W. (2001). The effect of injection speed on the perception of intramuscular injection pain. A clinical update. Retrieved on Oct 7, 2015, from http://www.ncbi.nlm.nih.gov/pubmed/11760527
National Institute for Occupational Safety and Health. (1999). Preventing needle stick injuries in the health care setting. Retrieved on Oct 5, 2015, from http://www.cdc.gov/niosh/docs/2000-108/pdfs/2000-108.pdf
National Institute of Health Clinical Center. (2015). NIH Clinical Center patient education material. Giving a subcutaneous injection. Retrieved on Oct 7, 2015, from http://www.cc.nih.gov/ccc/patient_education/pepubs/subq.pdf
National Patient Safety Agency. (2009). Safety in doses. Improving the use of medication in NHS. Retrieved on June 11, 2015, from http://www.nrls.npsa.nhs.uk/resources/?entryid45=61625
National Priority Partnership. (2010). Preventing medical errors: A $21 billion opportunity. Retrieved on June 3, 2015, from http://psnet.ahrq.gov/resource.aspx?resourceID=20529
Nisbet, A. C. (2006). Intramuscular gluteal injections in the increasingly obese population: Retrospective study. British Medical Journal, 332(7542), 637-638. doi: http://dx.doi.org/10.1136/bmj.38706.742731.47
Ogston-Tuck, S. (2014a). Intramuscular injection technique: An evidence-based approach. Nursing Standard, 29(4), 52-59. Retrieved on Oct 7, 2015, from http://journals.rcni.com/doi/pdfplus/10.7748/ns.29.4.52.e9183
Ogston-Tuck, S. (2014b). Subcutaneous injection technique: An evidence-based approach. Nursing Standard, 29(3), 53-58.
Perry, A., Potter, P., & Ostendorf, W. (2014). Clinical skills and nursing techniques (8th ed.). St Louis, MO: Elsevier-Mosby.
Prakash, V., Koczmara, C., Saveage, P., Trip, K., Stewart, J., Mccurdie, T., Cafazzo, J., & Trbovich, P. (2014). Mitigating errors caused by interruptions during medication verification and administration: Interventions in a simulated ambulatory chemotherapy setting. BMJ Quality and Safety, 0 (1-10). doi:10.1136/bmjqs-2013-002484
Pratt, J. R., Pellowe, C. M., Wilson, J. A., Loveday, H. P., Harper, P. J., Jones, S. L. R. J., McDougall, C., & Wilcox, M. J. (2007). Epic2: National evidence based guidelines for preventing health care associated infections in NHS hospitals in England. J Hosp Infect 65, Suppl 1, S1 – 64.
Provincial Infectious Disease Advisory Committee. (2014). Best practices for hand hygiene in all health care settings (4th ed.). Retrieved on June 4, 2015, from http://www.publichealthontario.ca/en/eRepository/2010-12%20BP%20Hand%20Hygiene.pdf
Rodgers, M.A., & King, L. (2000). Drawing up and administering intramuscular injections: A review of the literature. Journal of Advanced Nursing, 31(3), 574–582. doi: 10.1046/j.1365-2648.2000.01312.x
Sepah, Y., Samad, L., & Altaf, A. (2014). Aspiration in injections: Should we continue or abandon the practice? F1000Research, 3(157) doi:10.12688/f1000research.1113.1
Siegel, J.D., Rhinehart, E., Jackson, M., Chiarello, L., and the Healthcare Infection Control Practices Advisory Committee (2007). Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Healthcare Settings. Retrieved on Nov. 8, 2015, from http://www.cdc.gov/ncidod/dhqp/pdf/isolation2007.pdf
Sisson, H. (2015). Aspirating during the intramuscular injection procedure: A systematic literature review. Journal of Clinical Nursing, Sept 24 (17-18), 2368-2375. doi: 10.1111/jocn.12824
Wilburn, S. Q. (2004). Needlestick sharps injury and prevention. The Online Journal of Issues in Nursing. Retrieved on Oct 5, 2015, from http://www.nursingworld.org/MainMenuCategories/ANAMarketplace/ANAPeriodicals/OJIN/TableofContents/Volume92004/No3Sept04/InjuryPrevention.html
Wilburn, S. Q., & Eijkemans, G. (2004). Preventing needle stick injuries among health care workers. International Journal of Occupational and Environmental Health, 10(4), 451-456.
Workers Compensation Act. (2015). Occupational health and safety regulation. Retrieved on July 20, 2015, from http://www.bclaws.ca/Recon/document/ID/freeside/296_97_04
Workman, B. (1999). Safe injection techniques. Nursing standard, 13(39), 47-53.
World Health Organization. (2012). Course: Knowledge is the enemy of unsafe care. Retrieved on June 1, 2015, from http://who.int/patientsafety/education/curriculum/course7_handout.pdf