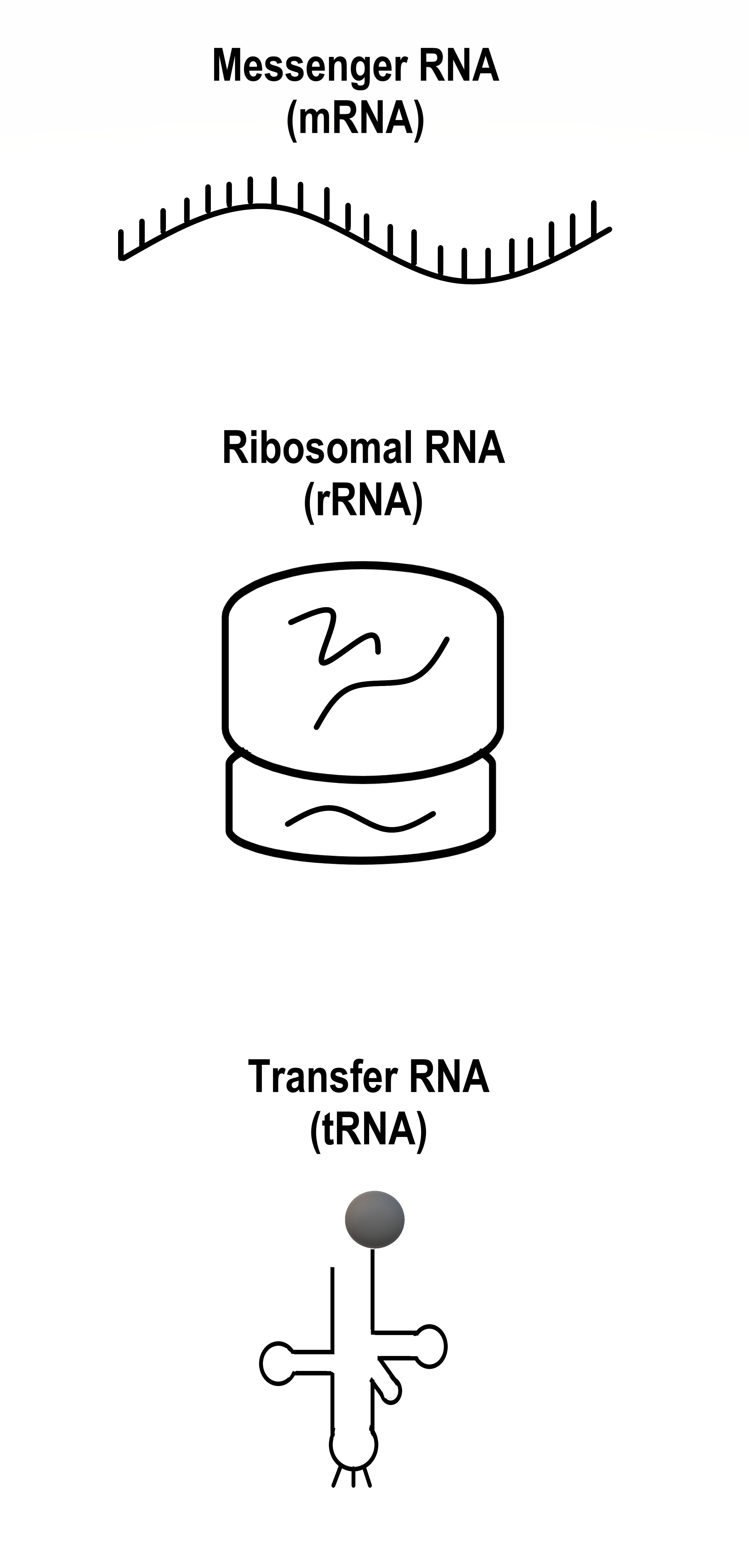
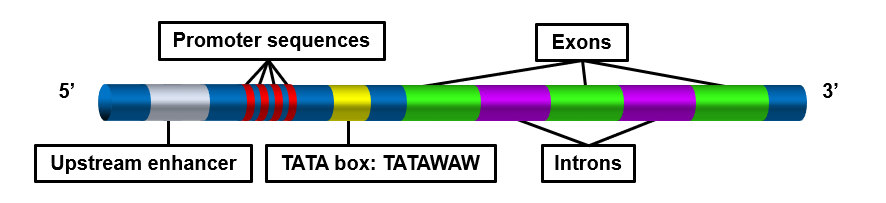
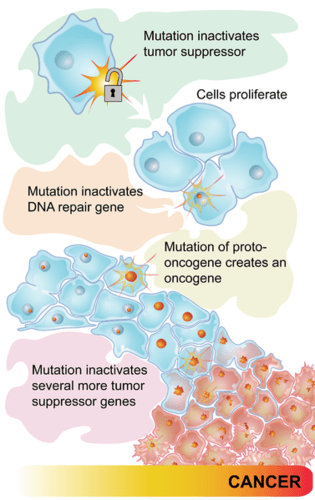
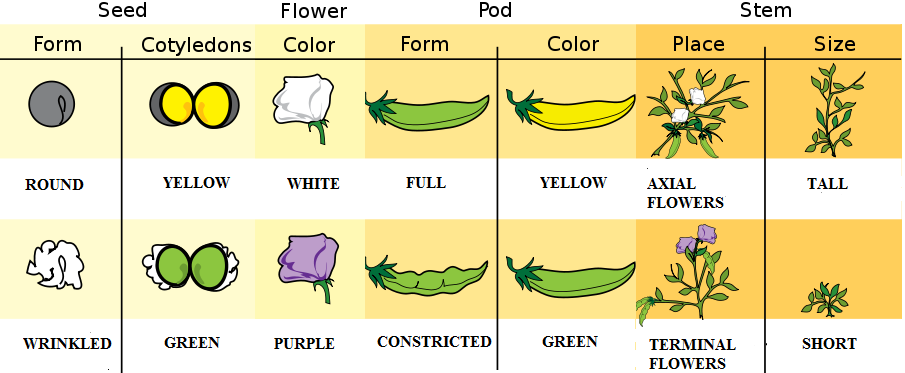
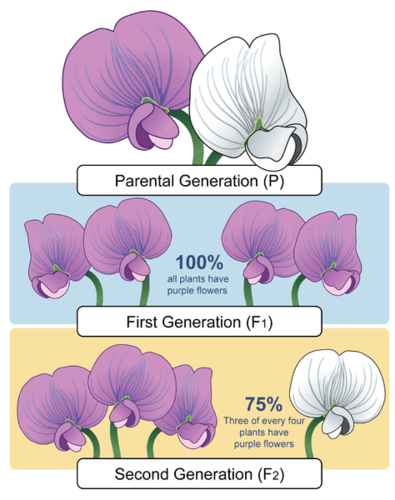
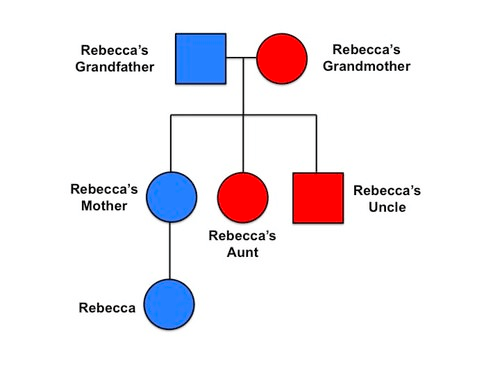
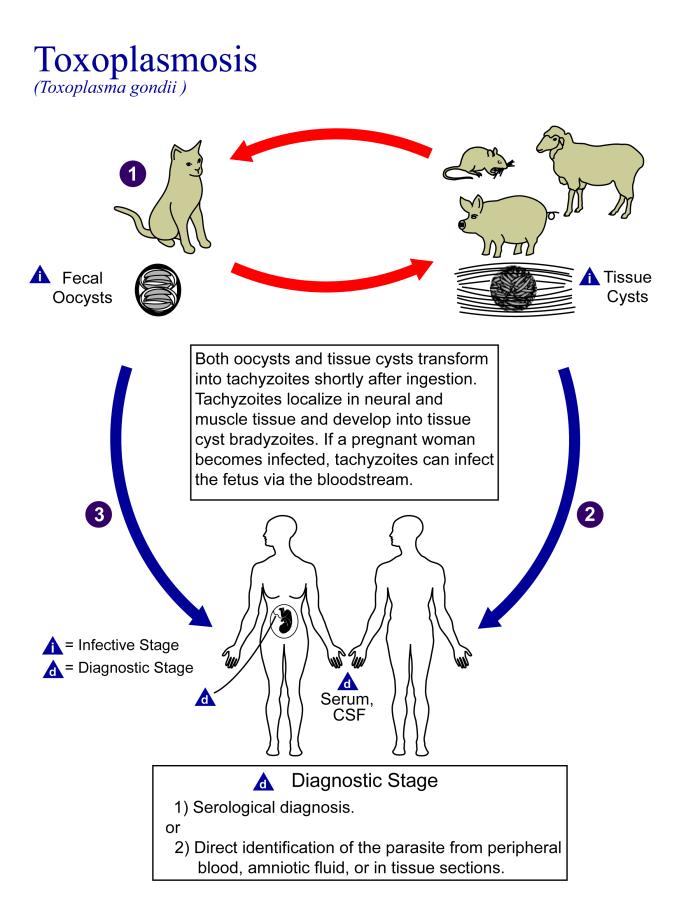
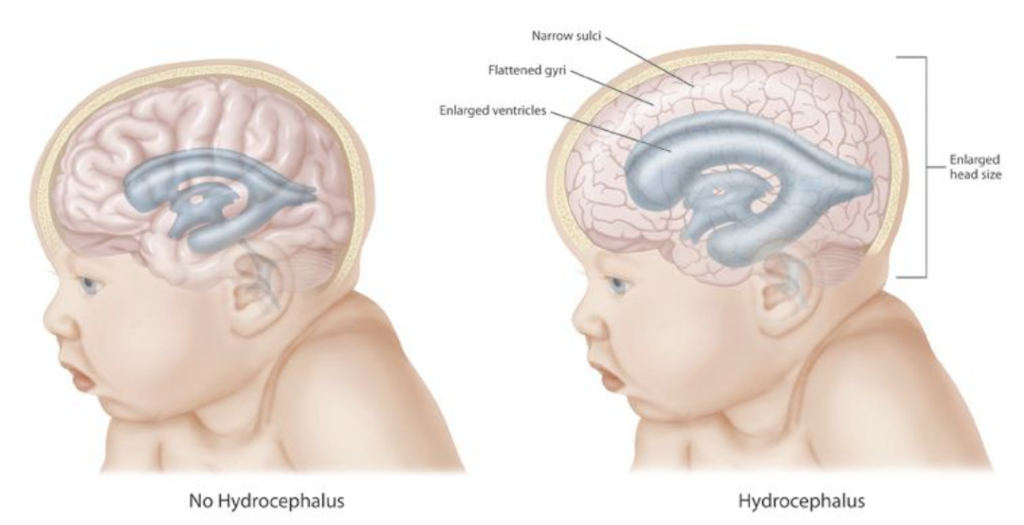
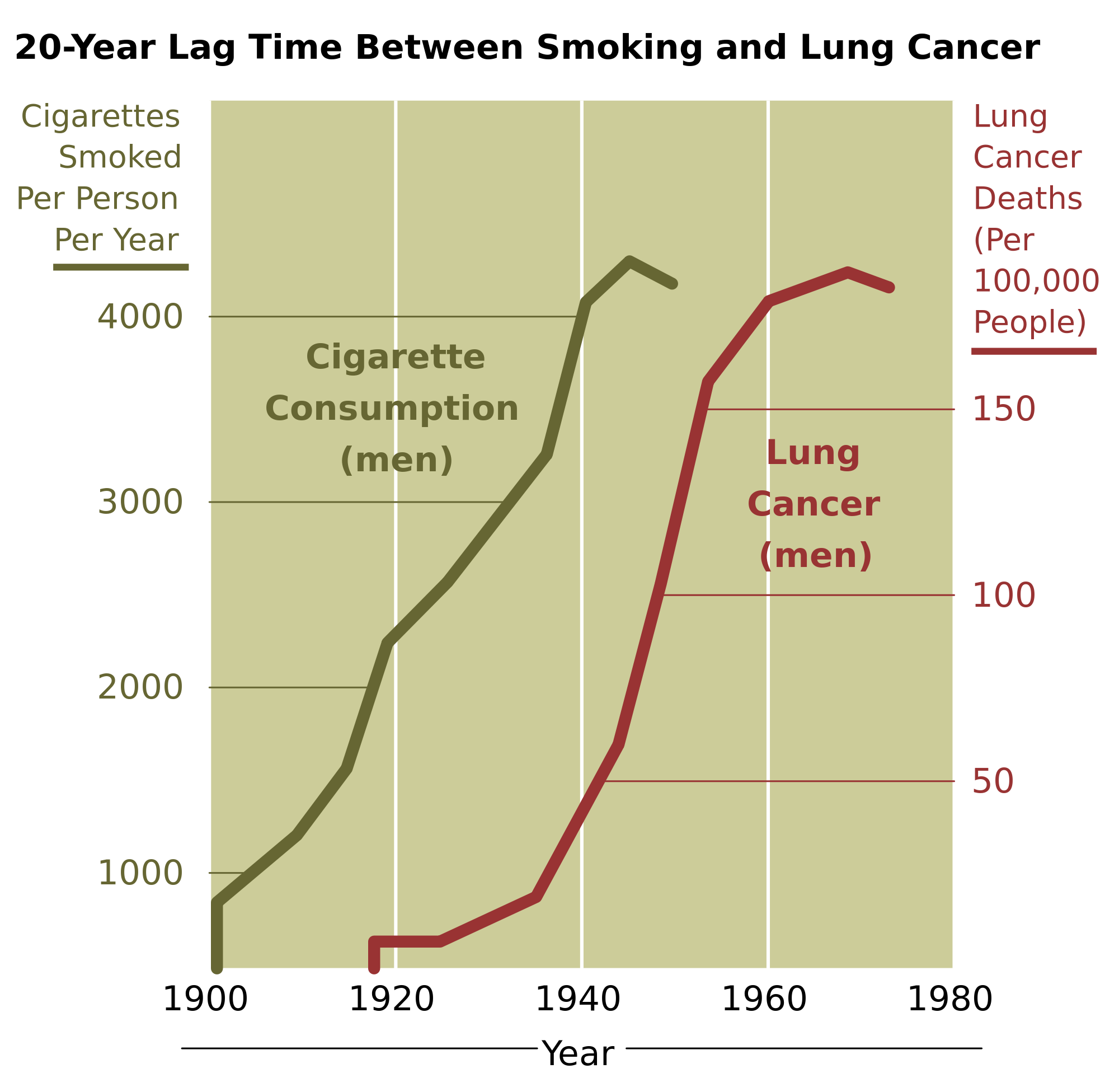
- 1N
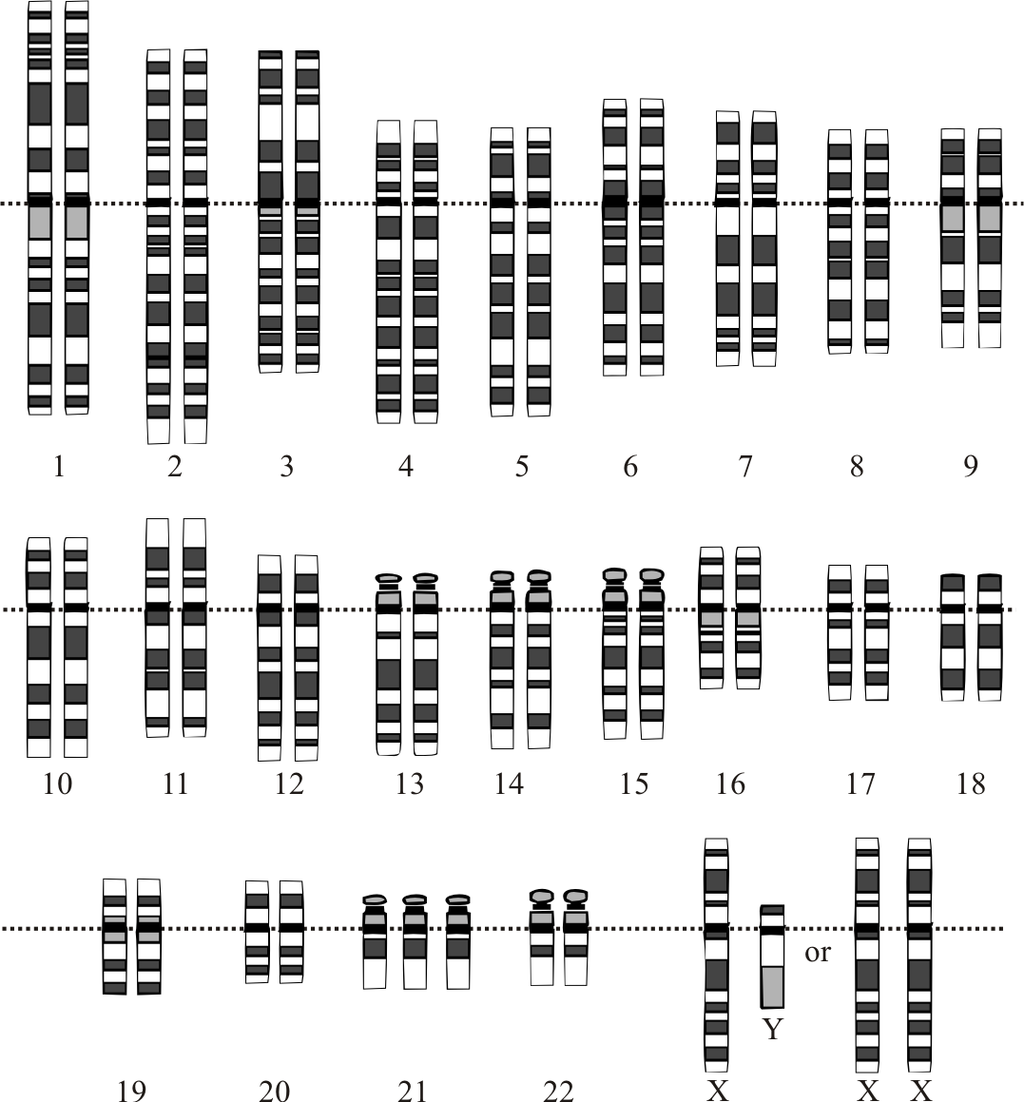
The term used when a cell has half the usual number of chromosomes.
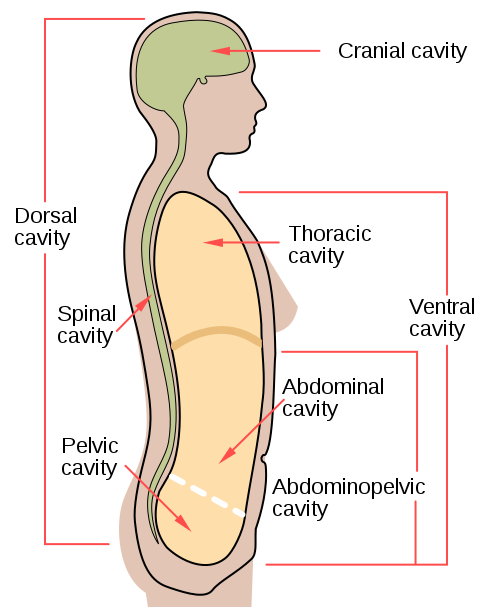
- Abdominal cavity
A large cavity found in the torso of mammals between the thoracic cavity, which it is separated from by the thoracic diaphragm, and the pelvic cavity. Organs of the abdominal cavity include the stomach, liver, gallbladder, spleen, pancreas, small intestine, kidneys, large intestine, and adrenal glands.
- Abdominopelvic cavities
Body cavity that fills the lower half of the trunk and holds the kidneys and the digestive and reproductive organs.
- Absorption
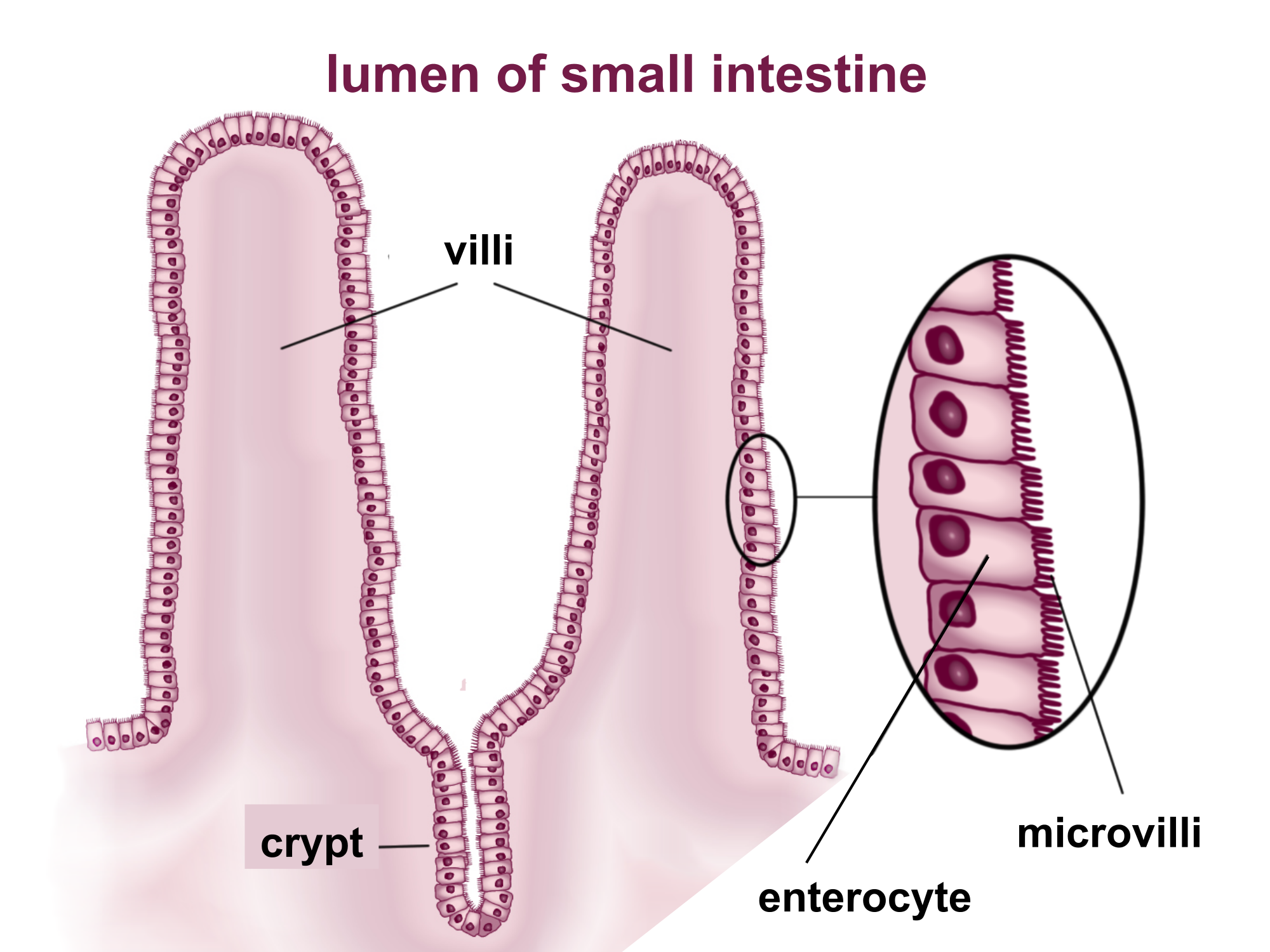
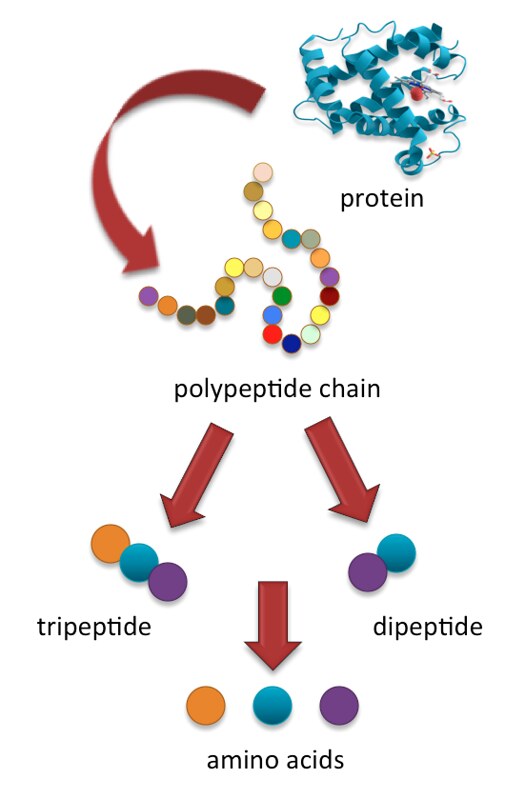
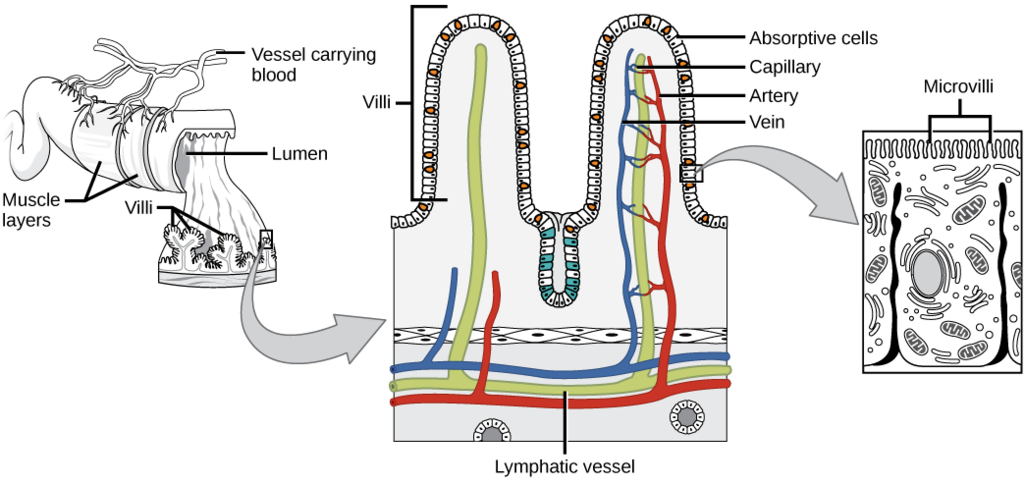
Process in which substances such as nutrients pass into the blood or lymph.
- Abstinence
The practice of refraining from some or all aspects of sexual activity.
- Acclimatization
The process in which an individual organism adjusts to a change in its environment, allowing it to maintain performance across a range of environmental conditions. Acclimatization occurs in a short period of time, and within the organism's lifetime.
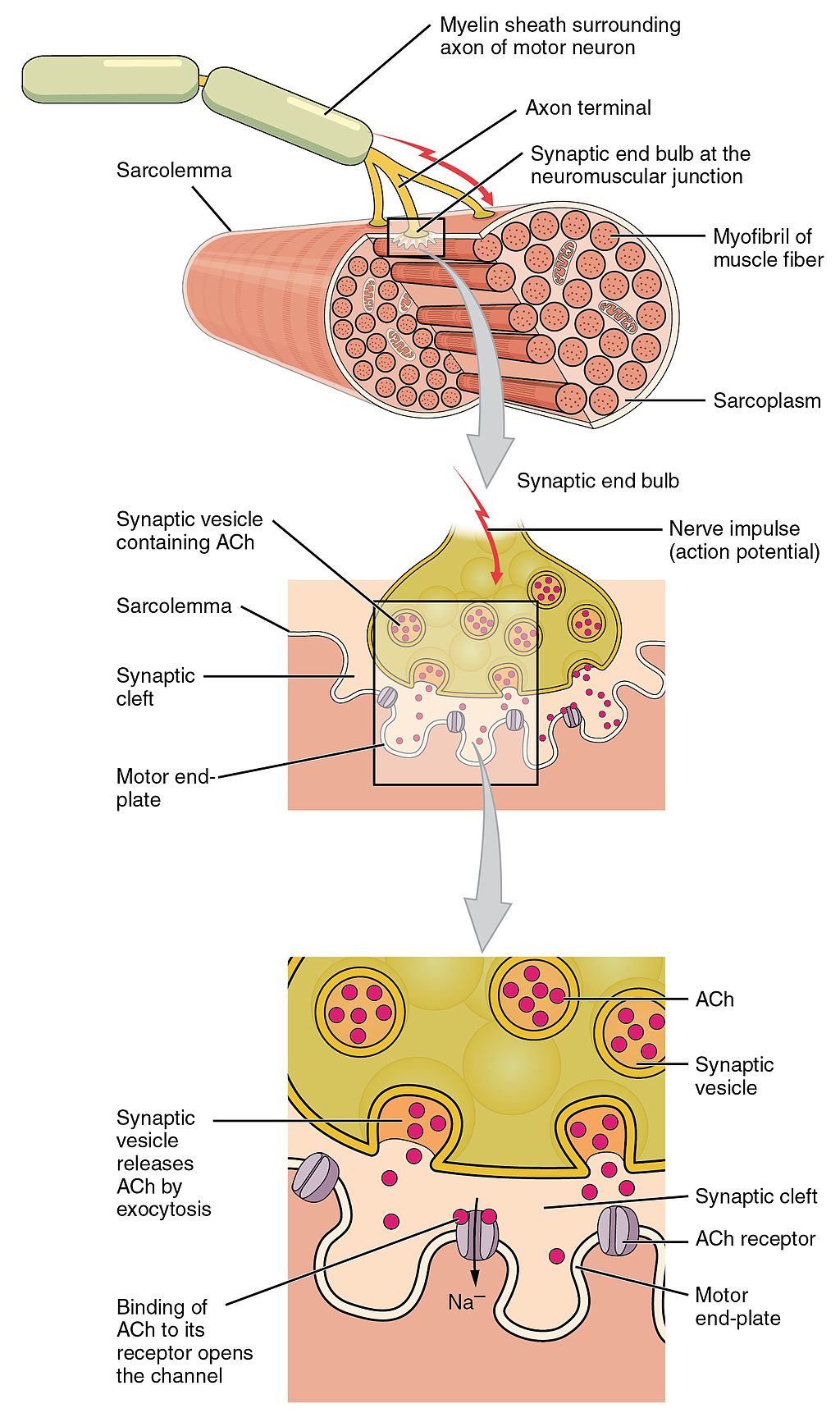
- Acetylcholine
An organic chemical that functions in the brain and body of many types of animals (and humans) as a neurotransmitter—a chemical message released by nerve cells to send signals to other cells, such as neurons, muscle cells and gland cells.
- Acid
An acid is a chemical substance which contains hydrogen and can react with other substances to form salts. Some acids burn or dissolve other substances that they come into contact with.
- Acidic
Having a higher proportion of hydronium ions than hydroxide ions; having the properties of an acid; having a pH below 7.
- Acidity
The level of acid in a substance.
- Acne
A common skin disorder in which pimples, blackheads, nodules, or other skin lesions occur when bacteria infect sebum-clogged pores.
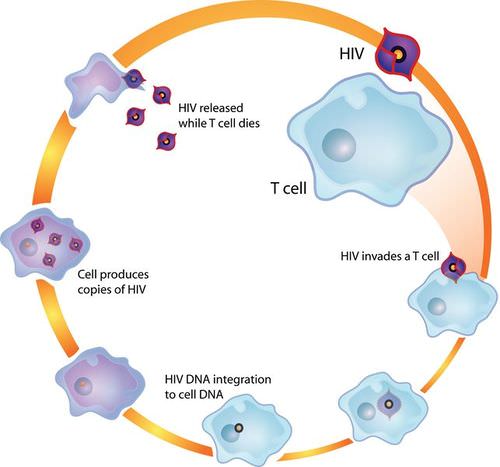
- Acquired immunodeficiency syndrome (AIDS)
The late stage of HIV infection that occurs when the body's immune system is badly damaged because of the virus.
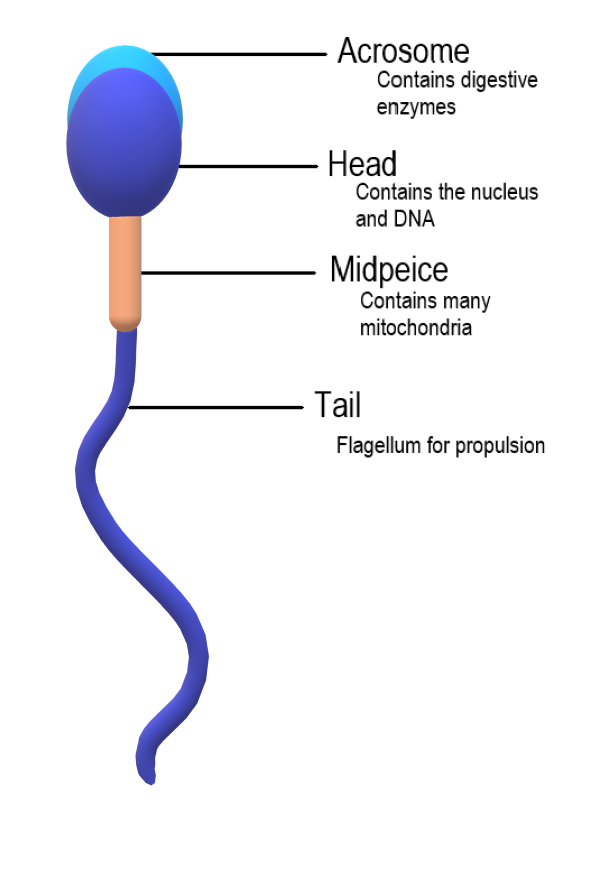
- Acrosome
An organelle covering the head of animal sperm and containing enzymes that digest the egg cell coating, thus permitting the sperm to enter the egg.
- Actin
A protein that forms (together with myosin) the contractile filaments of muscle cells, and is also involved in motion in other types of cells.
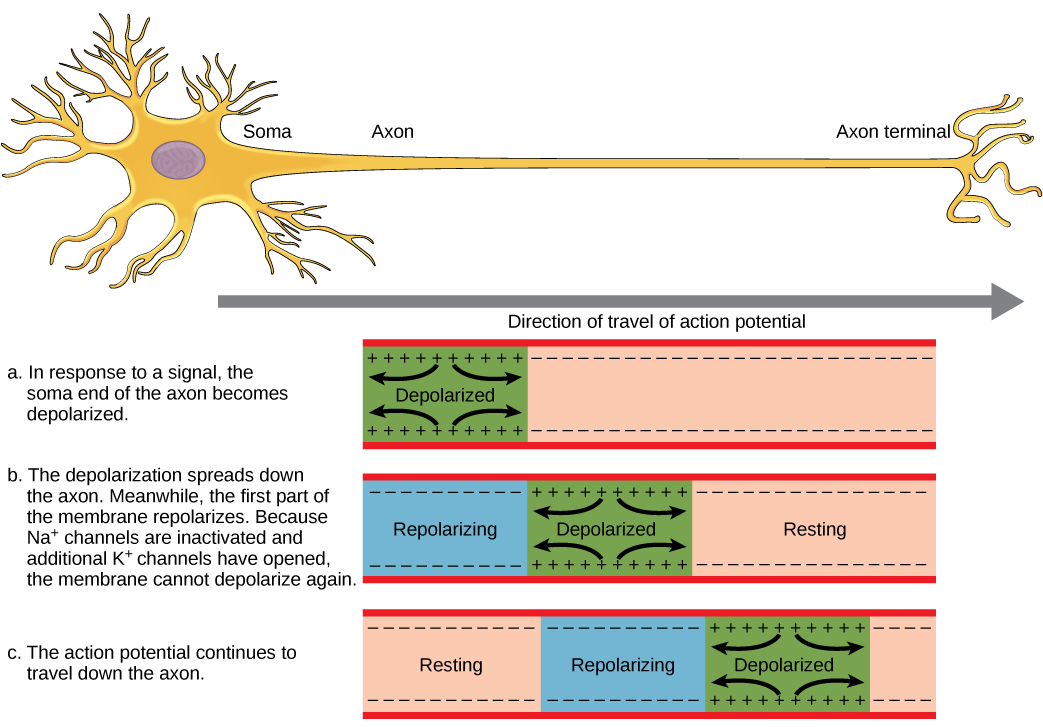
- Action potential
Reversal of electrical charge across the membrane of a resting neuron that travels down the axon of the neuron as a nerve impulse.
- Activation energy
The minimum energy required to cause a reaction to occur.
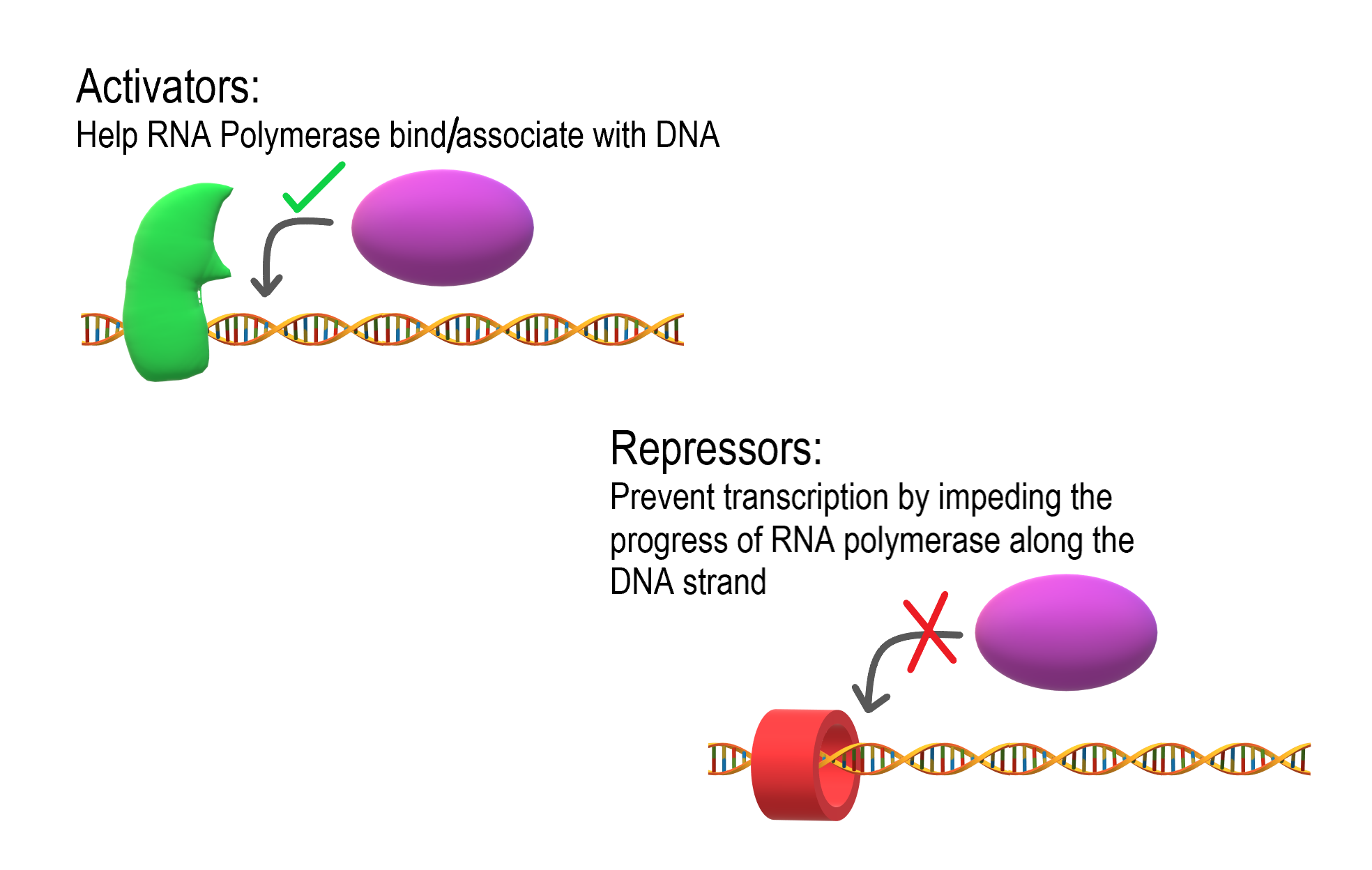
- Activators
Regulatory proteins that promote transcription by enhancing the interaction of RNA polymerase with the promoter.
- Active immunity
The ability to resist a specific pathogen that results when an adaptive immune response to the pathogen produces memory lymphocytes for that pathogen.
- Active transport
The movement of ions or molecules across a cell membrane into a region of higher concentration, assisted by enzymes and requiring energy.
- Adaptation
A genetically-based trait that has evolved because it helps living things survive and reproduce in a given environment.
- Adaptive immune system
A subset of the immune system that makes tailored attacks against specific pathogens or tumor cells such as the production of antibodies that match specific antigens.
- Addiction
The compulsive use of a drug, despite negative consequences that such use may entail.
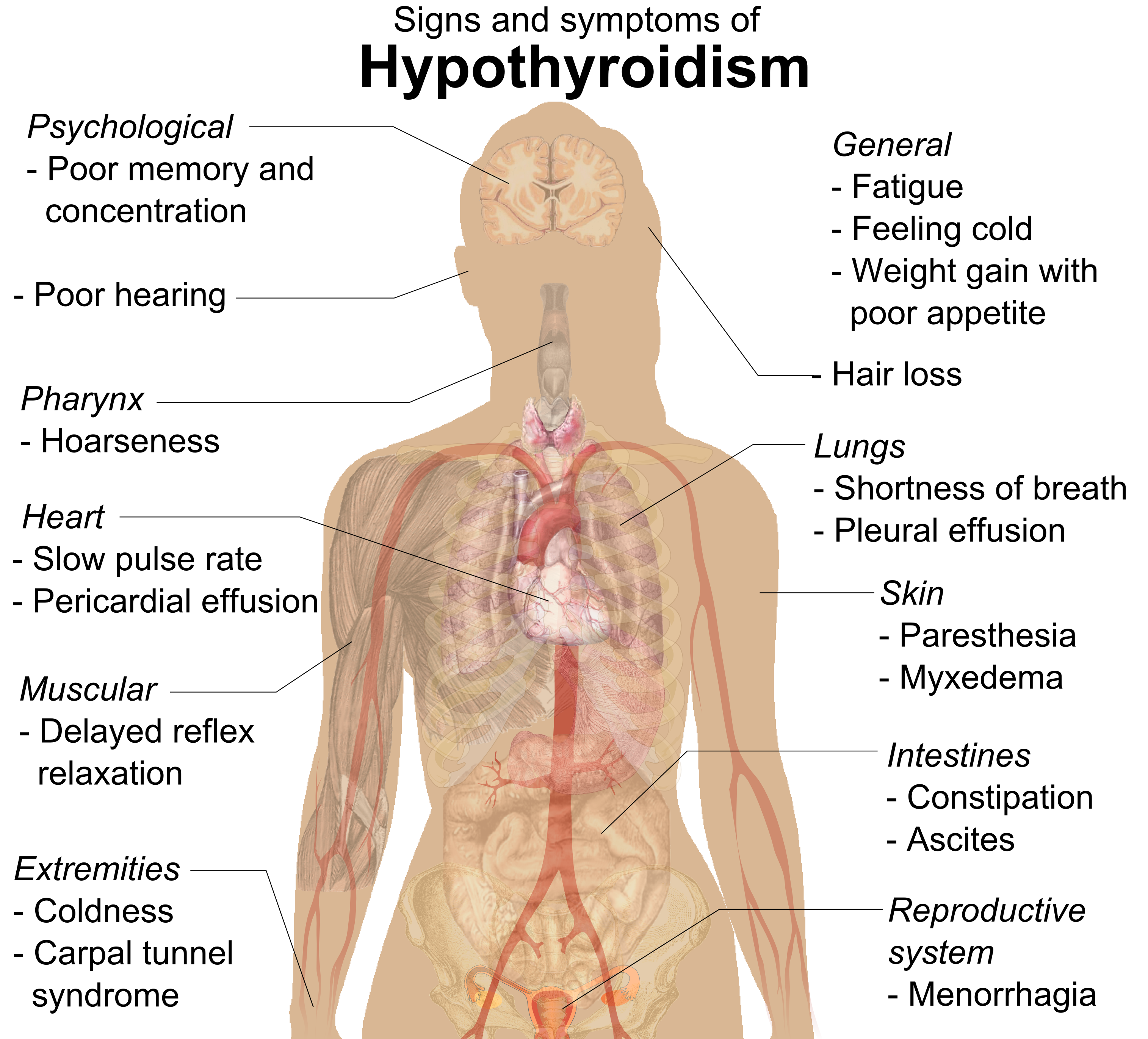
- Addison’s disease
A disorder characterized by hyposecretion of the adrenal cortex hormone cortisol, generally because the immune system attacks and destroys the adrenal gland.
- Admixture
The presence of DNA in an individual from a distantly-related population or species, as a result of interbreeding between populations or species who have been reproductively isolated and genetically differentiated. Admixture results in the introduction of new genetic lineages into a population.
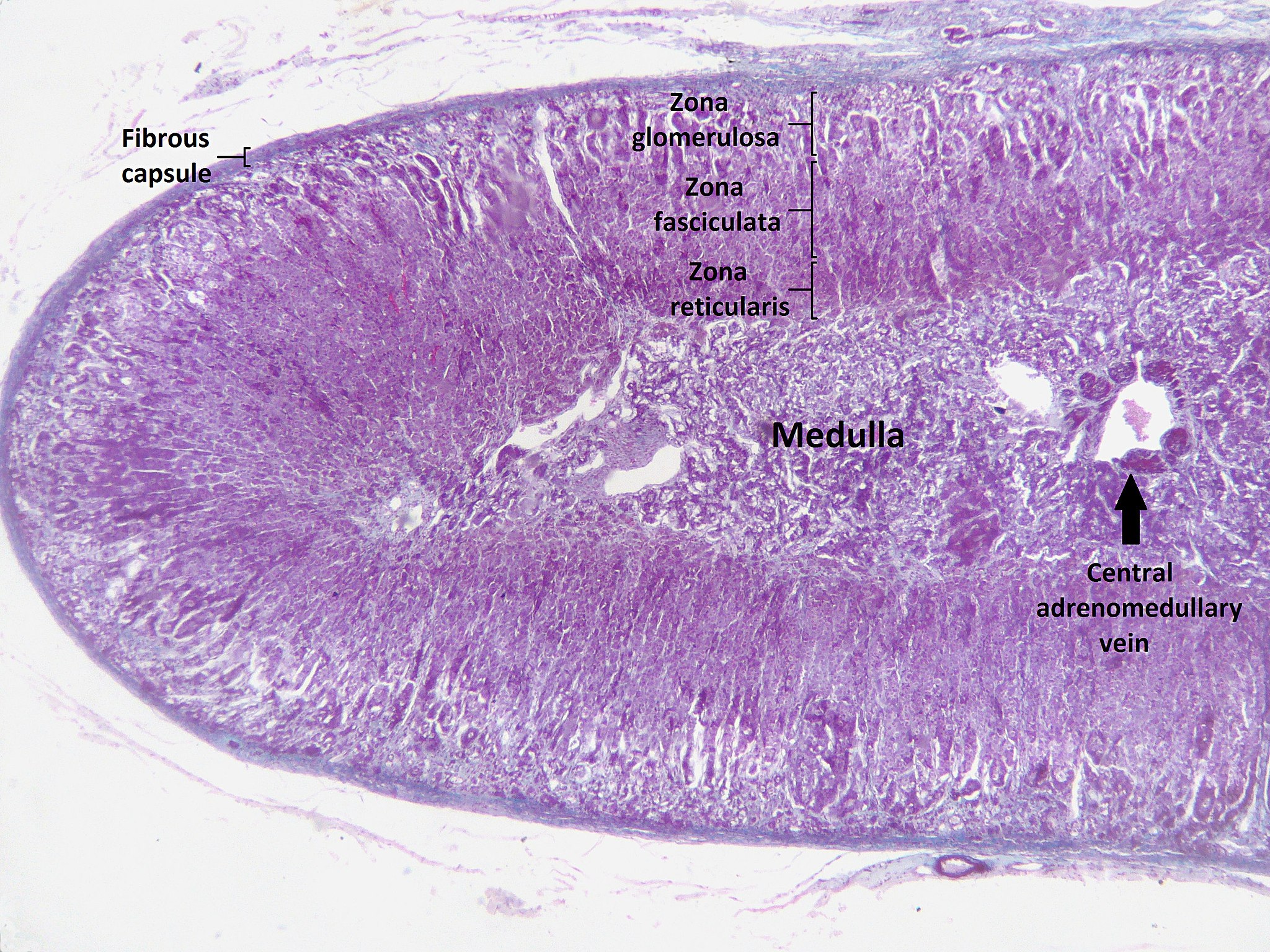
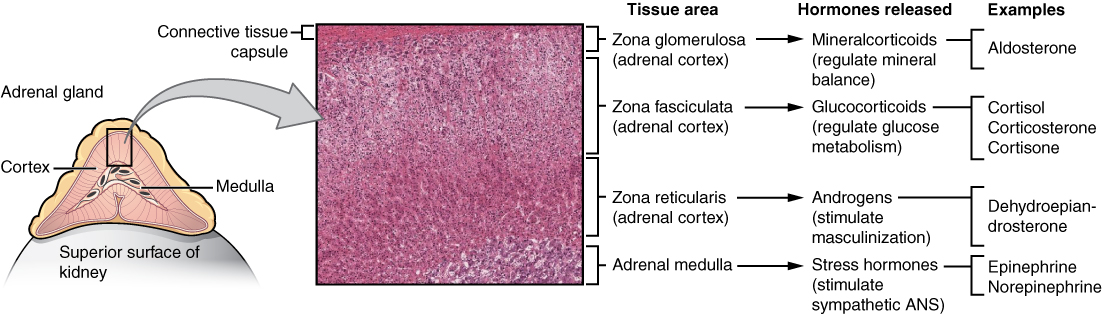
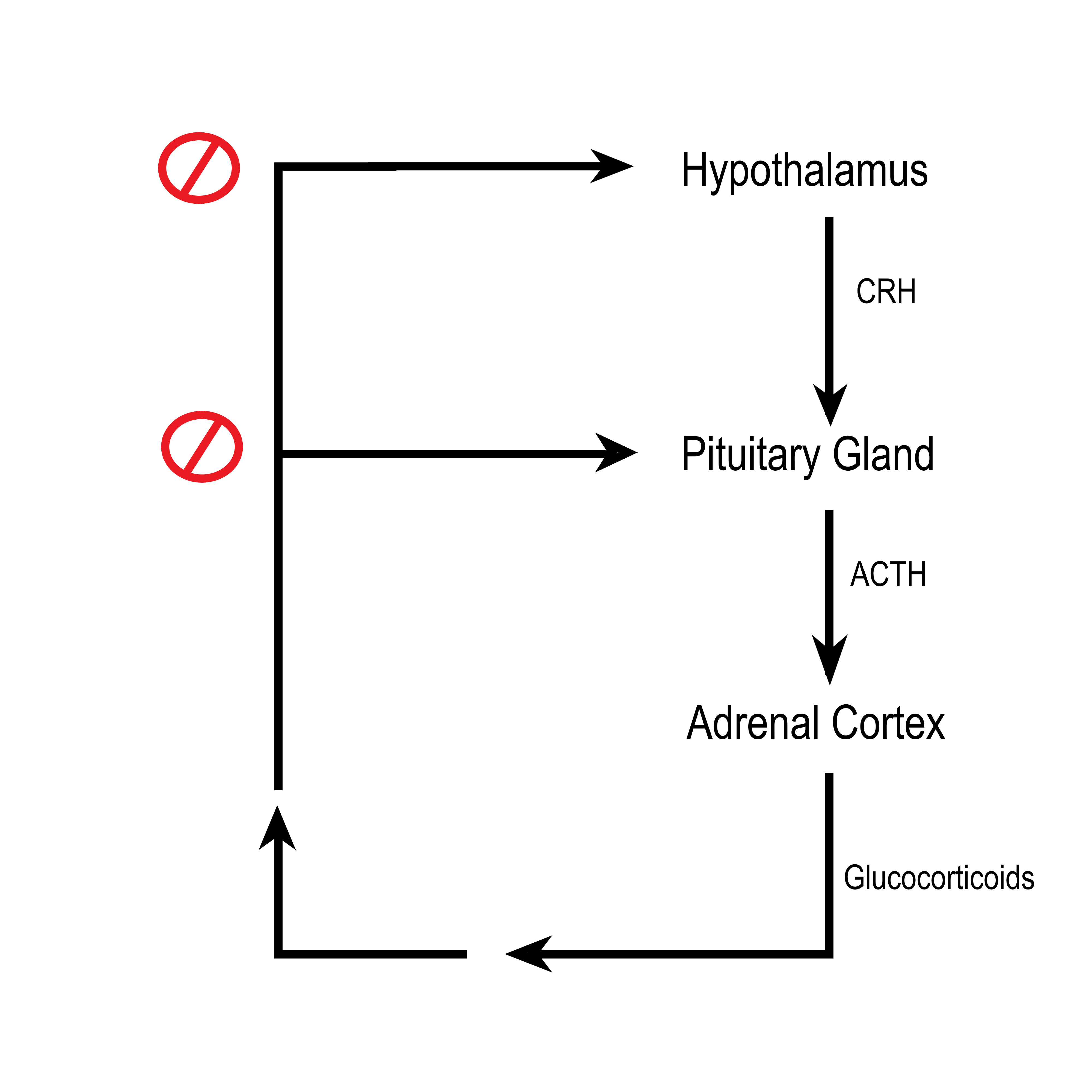
- Adrenal cortex
The outer layer of the adrenal gland that produces steroid hormones such as cortisol and aldosterone.
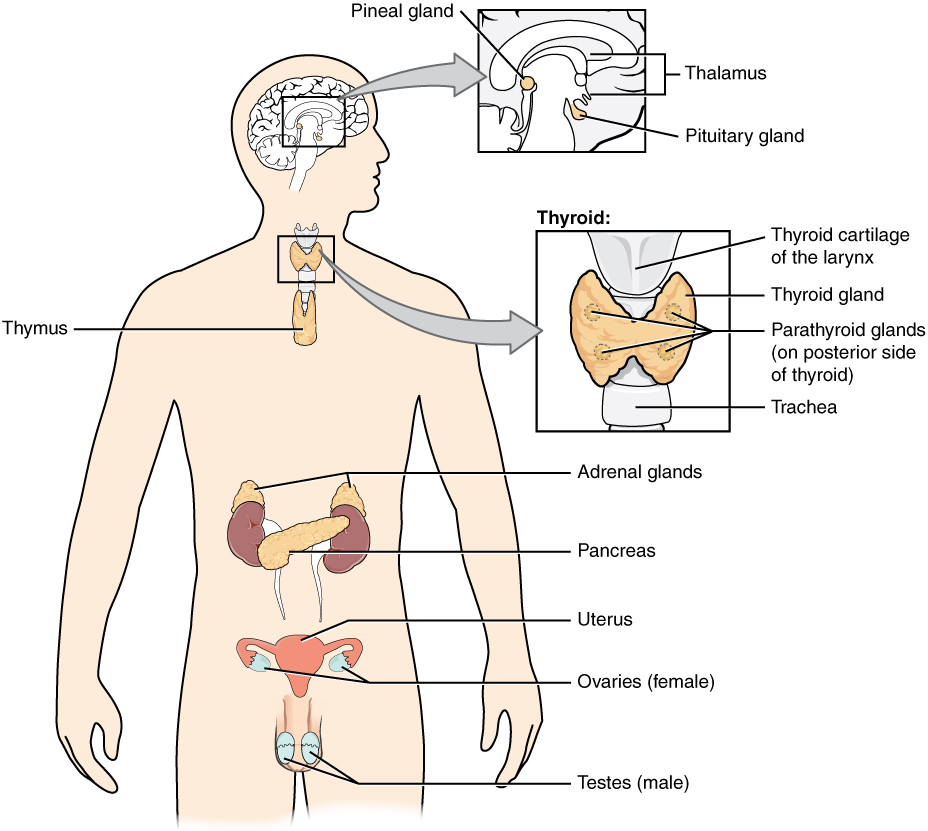
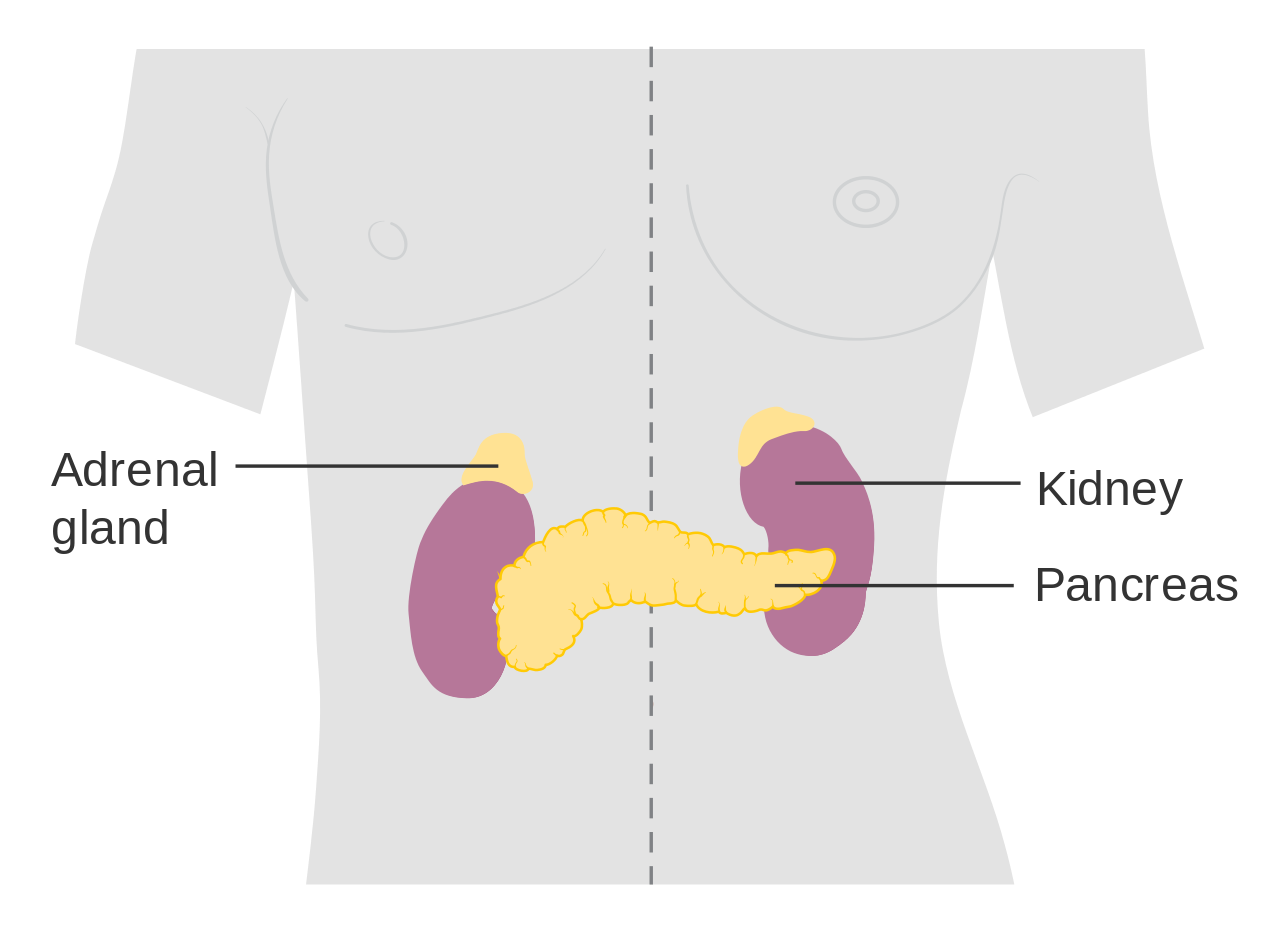
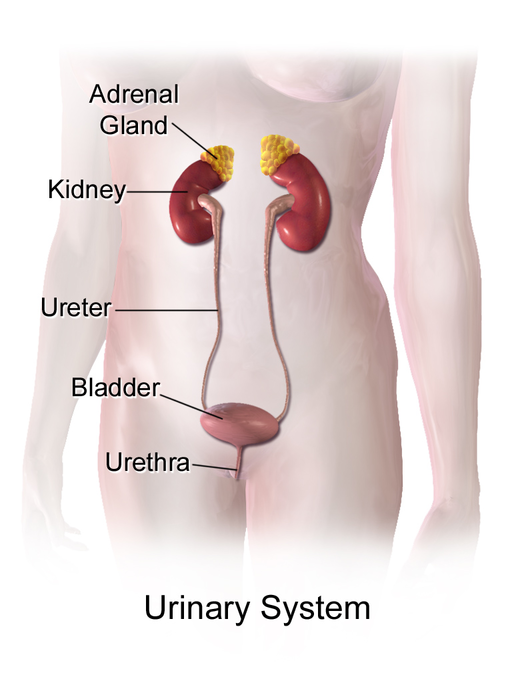
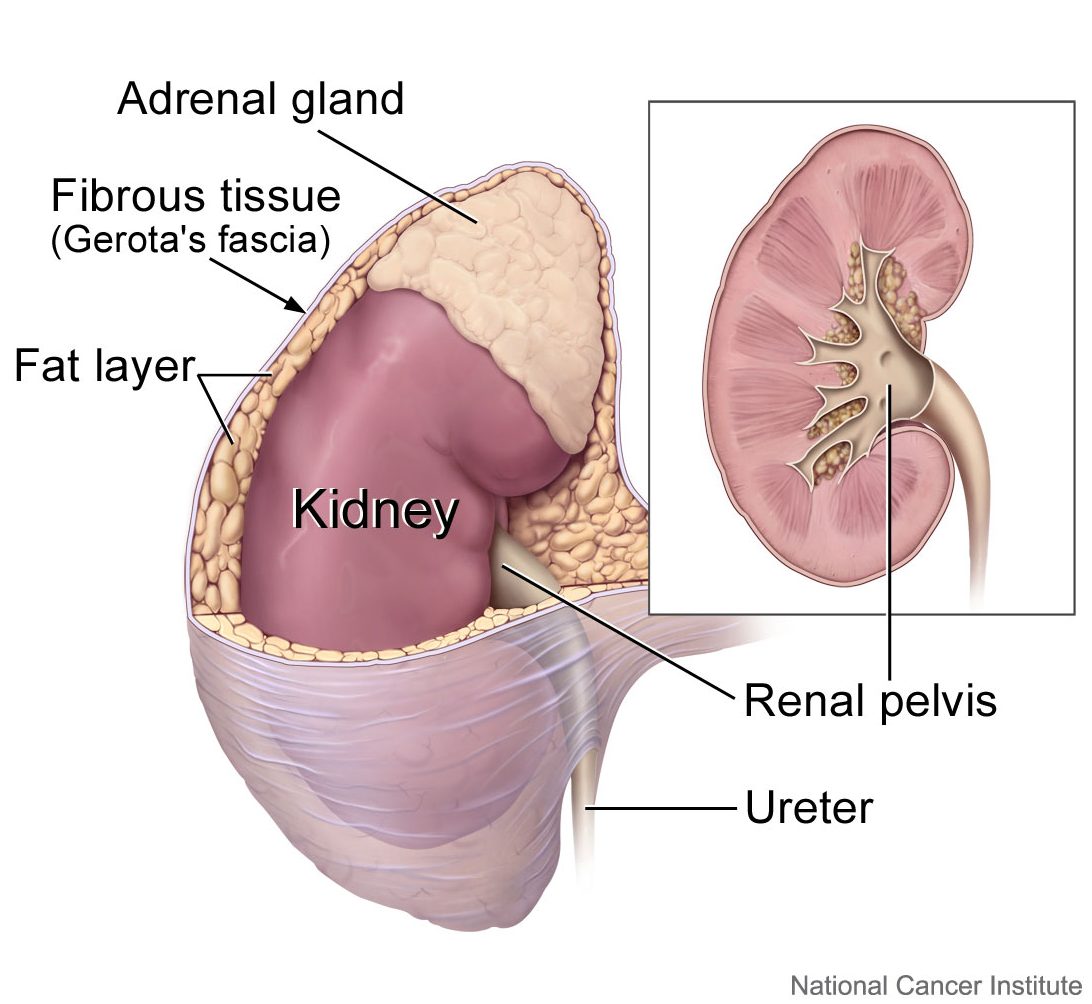
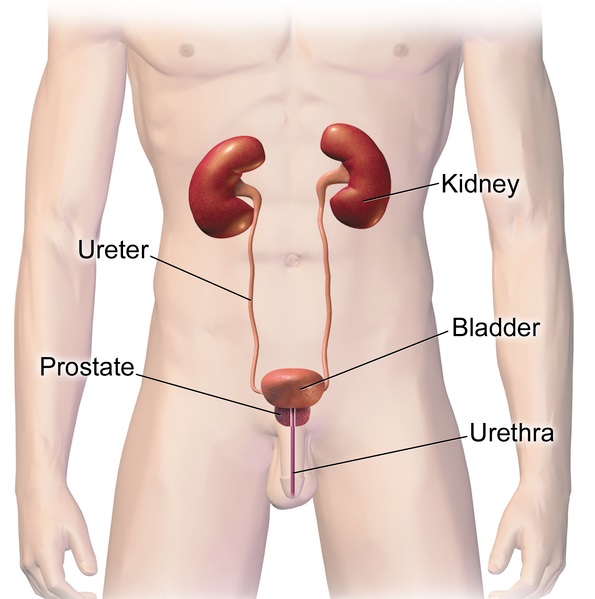
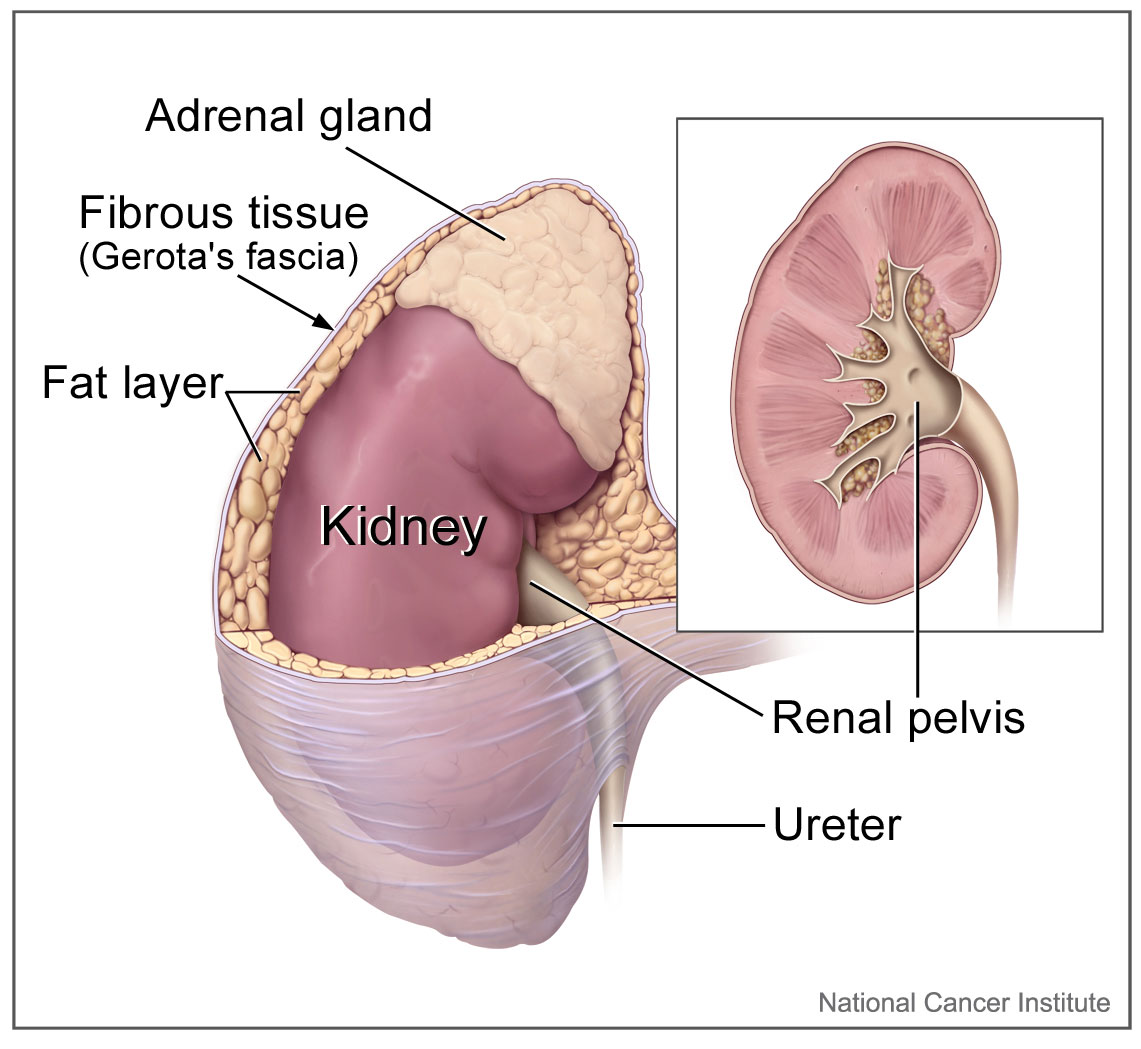
- Adrenal gland
one of a pair of glands located on top of the kidneys that secretes hormones such as cortisol and adrenaline
- Adrenal medulla
The central part of an adrenal gland that is surrounded by the adrenal cortex and that produces catecholamine hormones including adrenaline.
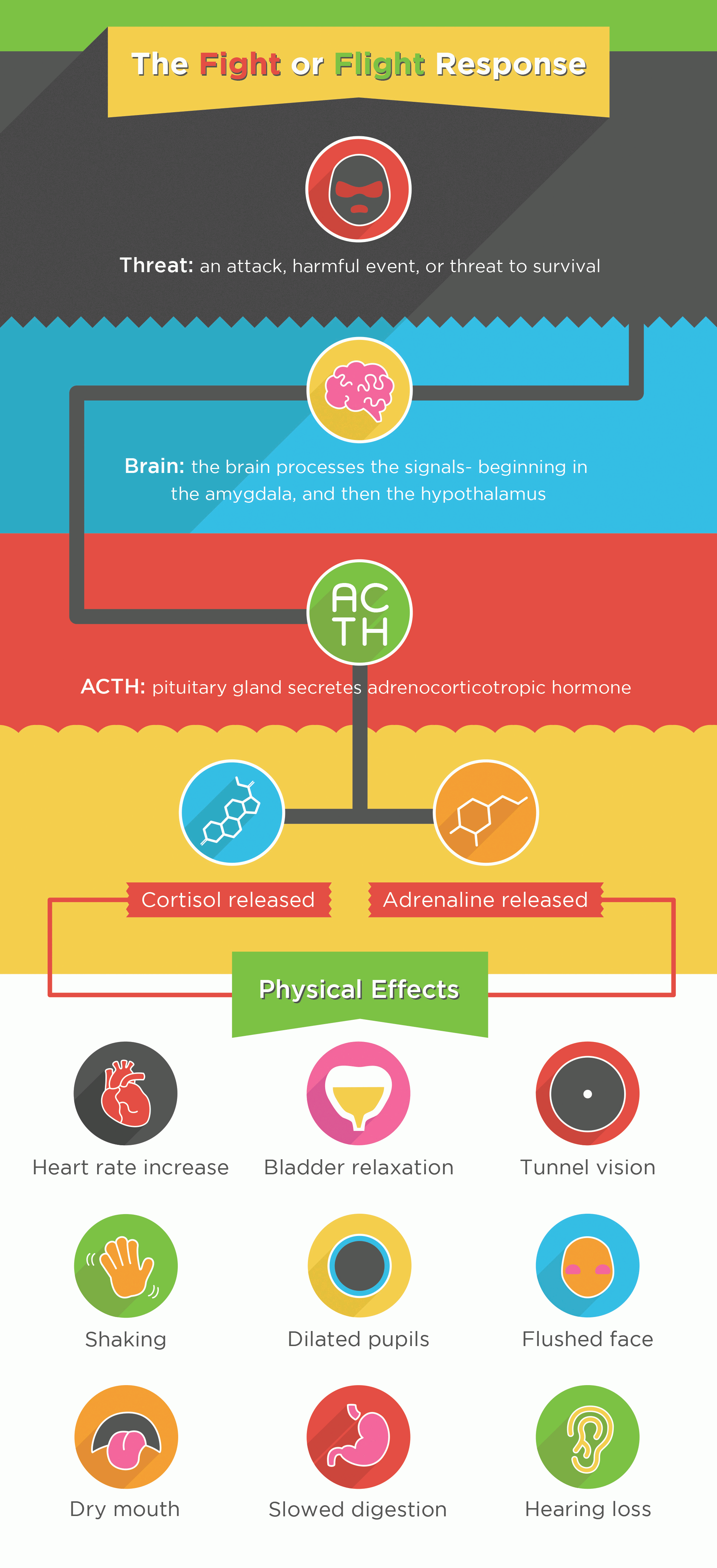
- Adrenaline
A non-steroid catecholamine hormone produced by the medulla of the adrenal glands that stimulates the fight-or-flight response.
- Aerobic
Relating to, involving, or requiring free oxygen.
- Aerobic exercise
Any physical activity in which muscles are used well below their maximum contraction strength but for a relatively long period of time, consuming a large amount of oxygen.
- Aerobic respiration
The process of producing cellular energy involving oxygen. Cells break down food in the mitochondria in a long, multi-step process that produces roughly 36 ATP. The first step in is glycolysis, the second is the Krebs cycle and the third is the electron transport system.
- Agonists
A drug that increases the activity or effect of a neurotransmitter.
- AIDS
Acquired Immunodeficiency Syndrome - a chronic, potentially life-threatening condition caused by the human immunodeficiency virus (HIV). By damaging your immune system, HIV interferes with your body's ability to fight infection and disease.
- Alcoholic fermentation
A biological process which converts sugars such as glucose, fructose, and sucrose into cellular energy, producing ethanol and carbon dioxide as by-products.
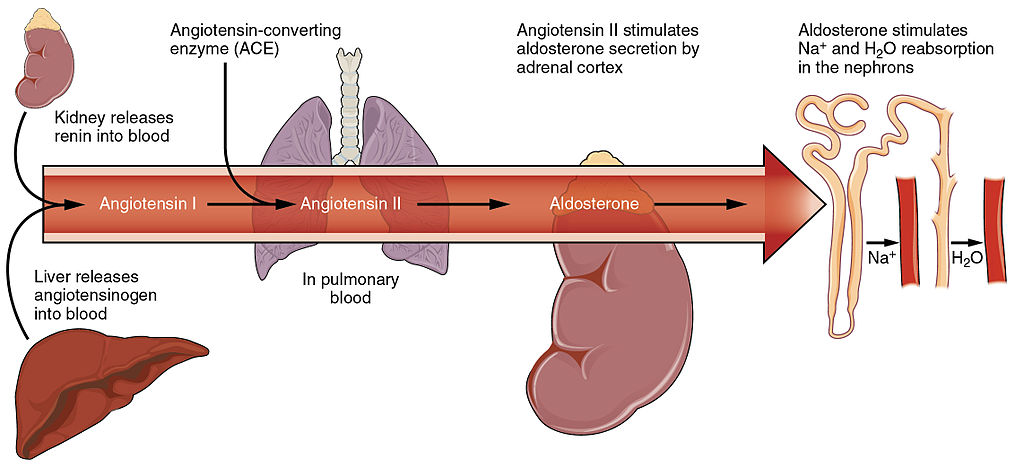
- Aldosterone
The main mineralocorticoid hormone which is responsible for sodium conservation in the kidney, salivary glands, sweat glands and colon.
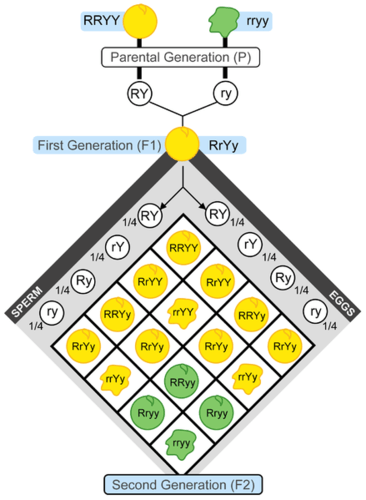
- Allele
A variant form of a given gene, meaning it is one of two or more versions of a known mutation at the same place on a chromosome. It can also refer to different sequence variations for a several-hundred base-pair or more region of the genome that codes for a protein.
- Allen’s rule
The principle holding that in a warm-blooded animal species having distinct geographic populations, the limbs, ears, and other appendages of the animals living in cold climates tend to be shorter than in animals of the same species living in warm climates.
- Allergen
Any substance, typically an antigen, that causes an allergy.
- Allergies
A damaging immune response by the body to a substance, especially pollen, fur, a particular food, or dust, to which it has become hypersensitive.
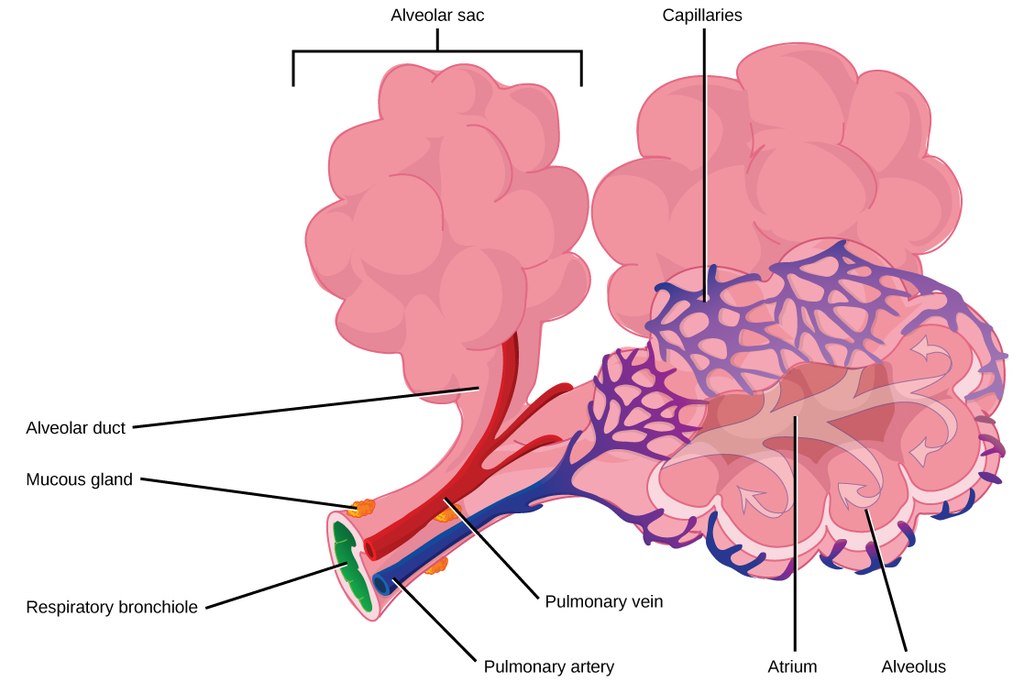
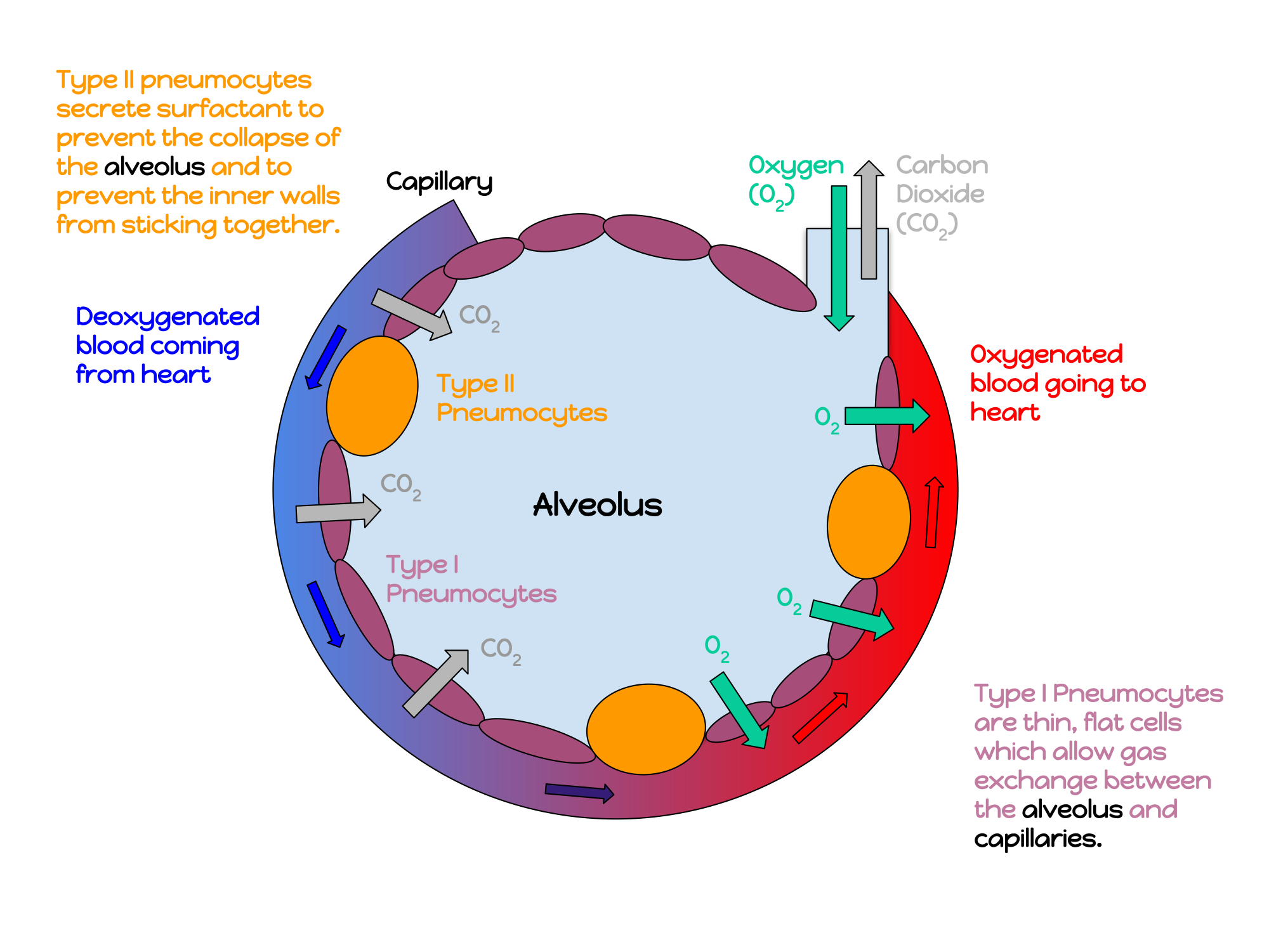
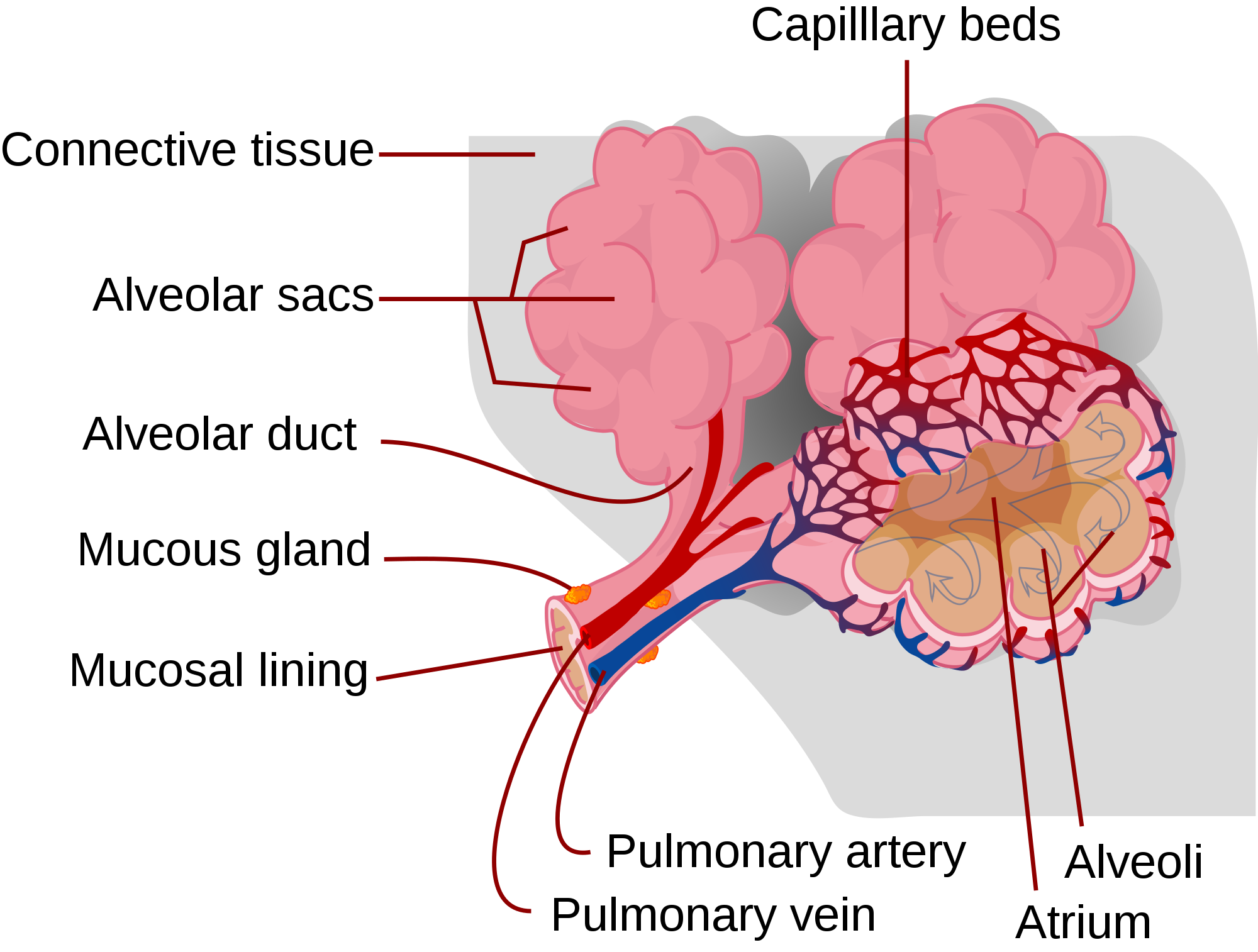
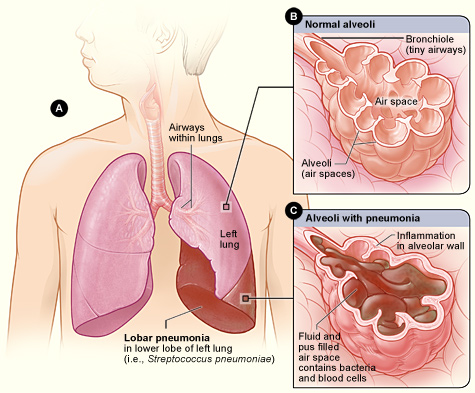
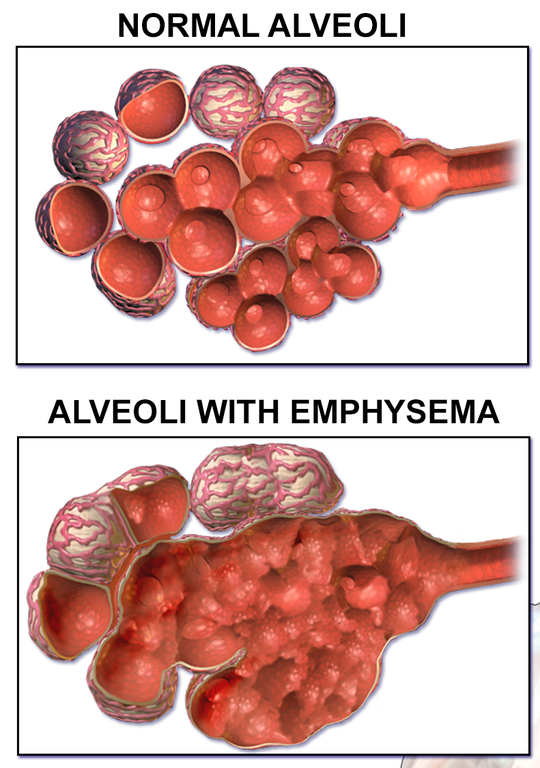
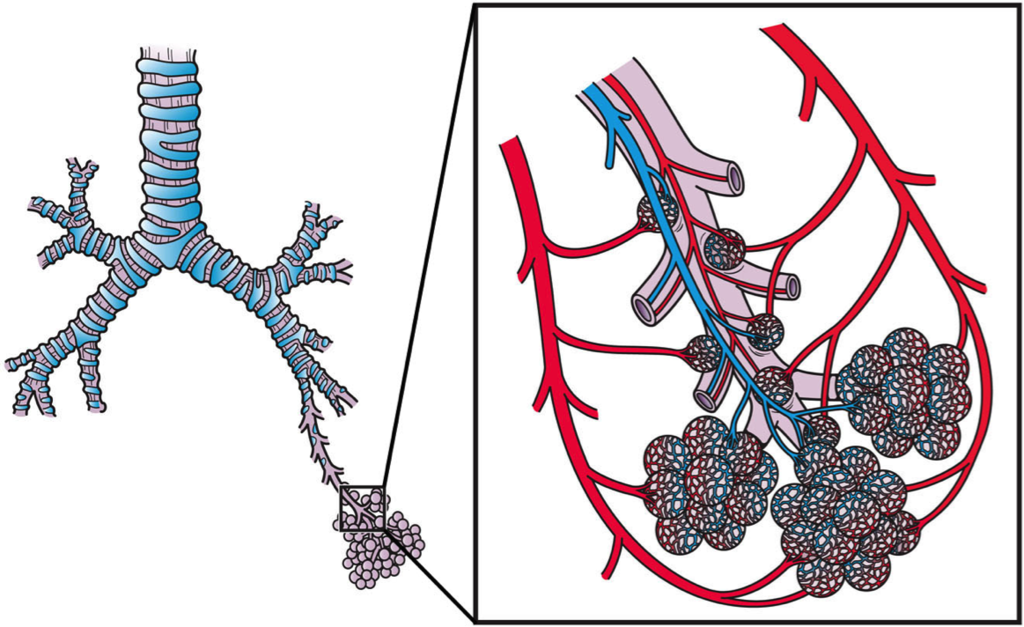
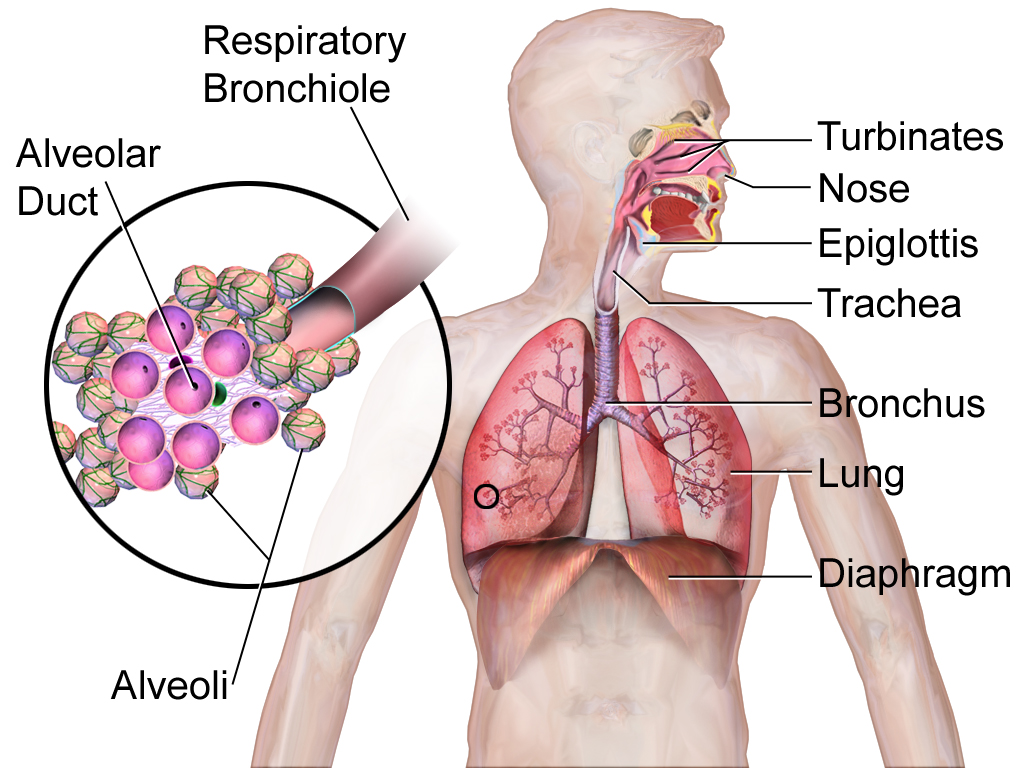
- Alveolus (plural, alveoli)
One of a cluster of tiny sacs at the ends of bronchioles in the lungs where pulmonary gas exchange takes place.
- Amino acid
Amino acids are organic compounds that combine to form proteins.
- Ammonia
A compound of nitrogen and hydrogen with the formula NH3. It is a common nitrogenous waste produced by the breakdown of amino acids in various cells in the body.
- Amniocentesis
A medical procedure used primarily in prenatal diagnosis of chromosomal abnormalities and fetal infections as well as for sex determination. In this procedure, a small amount of amniotic fluid, which contains fetal tissues, is sampled from the amniotic sac surrounding a developing fetus.
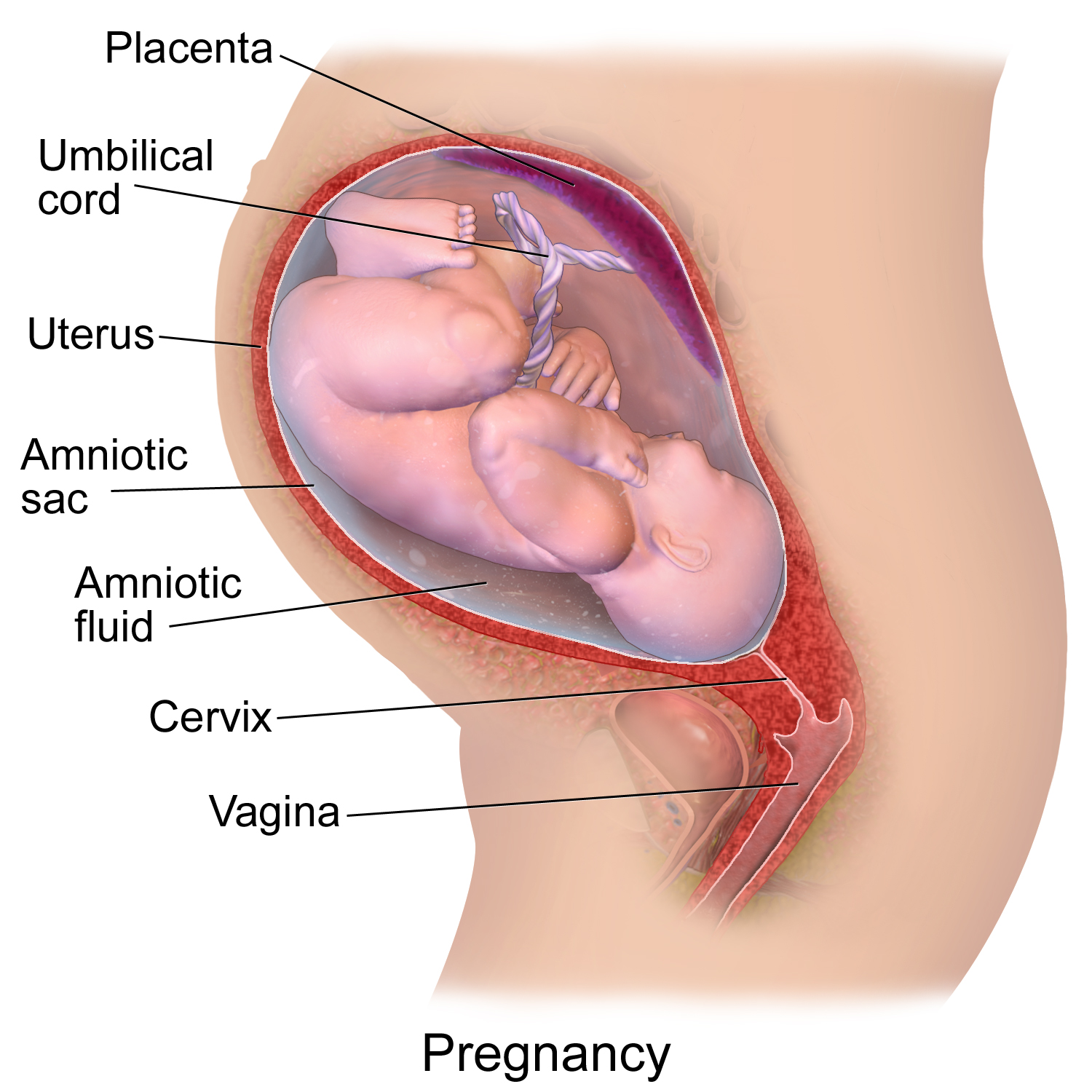
- Amniotic sac
The fluid-filled sac that contains and protects a fetus in the womb.
- Amygdala
A roughly almond-shaped mass of gray matter inside each cerebral hemisphere, involved with the experiencing of emotions. Responsible for the perception of emotions such as anger, fear, and sadness, as well as the controlling of aggression. The amygdala helps to store memories of events and emotions so that an individual may be able to recognize similar events in the future.
- Amylase
An enzyme, found chiefly in saliva and pancreatic fluid, that converts starch and glycogen into simple sugars.
- Anabolic reaction
Anabolic reactions are endergonic, meaning they require an input of energy to progress and are not spontaneous. They involve creation of larger molecules from smaller units.
- Anabolic steroid
A synthetic steroid hormone that resembles testosterone in promoting the growth of muscle. Such hormones are used medicinally to treat some forms of weight loss and (illegally) by some athletes and others to enhance physical performance.
- Anabolism
Synthesis of larger molecules from smaller ones.
- Anaerobic
Carried out in or pertaining to the absence of oxygen.
- Anaerobic exercise
Any physical activity in which muscles are used at close to their maximum contraction strength but for a relatively short period to time, consuming a small amount of oxygen.
- Anaerobic respiration
Respiration using electron acceptors other than molecular oxygen. Although oxygen is not the final electron acceptor, the process still uses a respiratory electron transport chain.
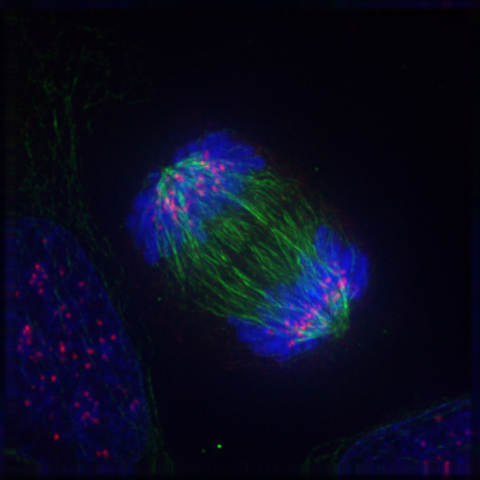
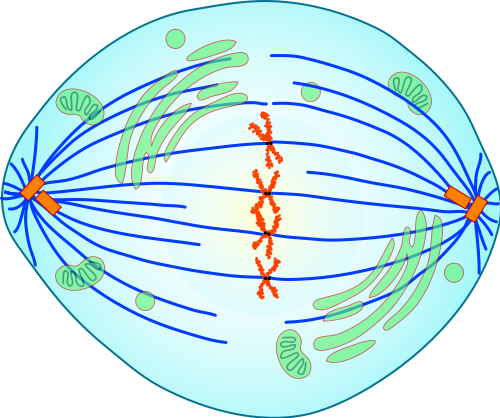
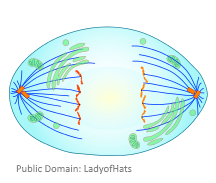
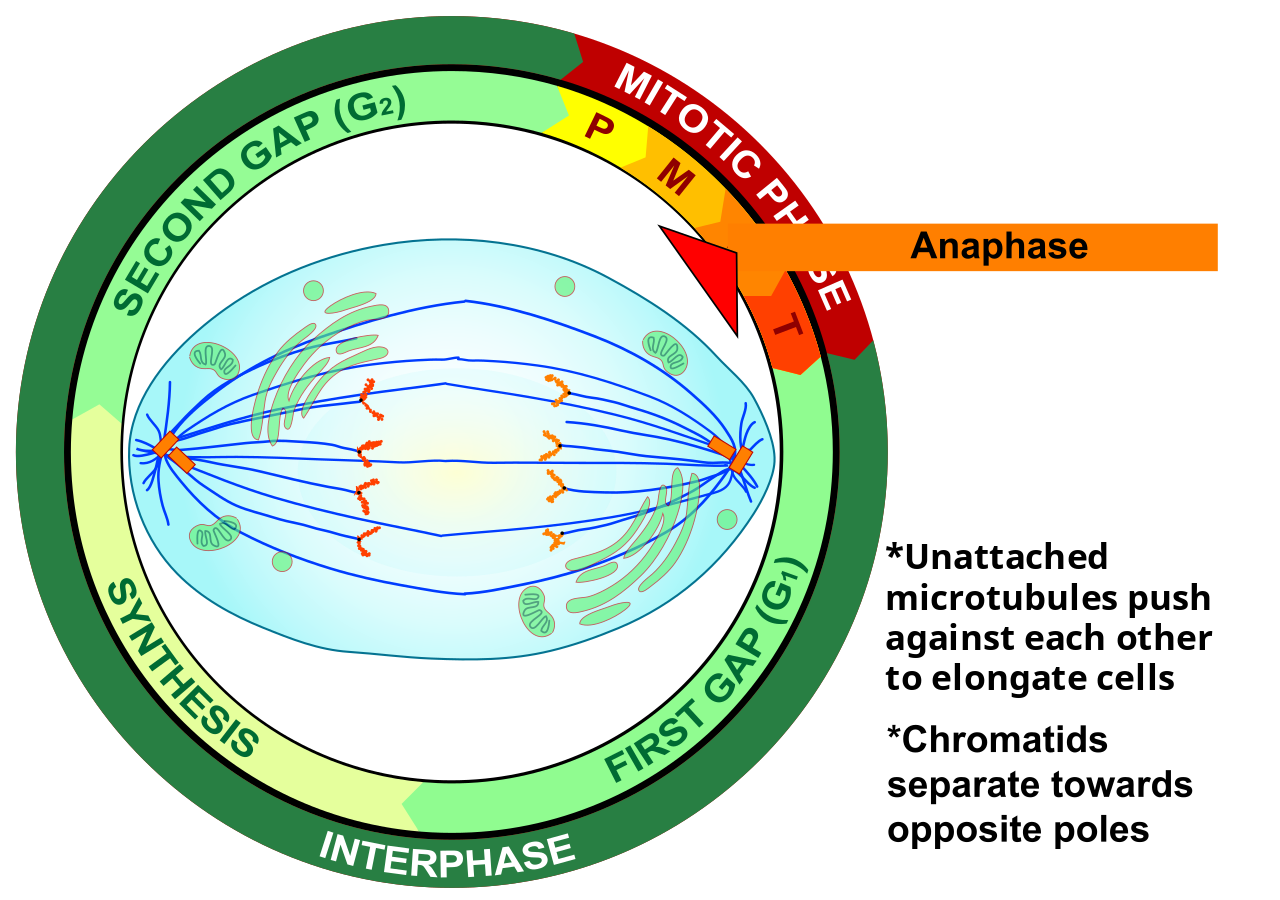
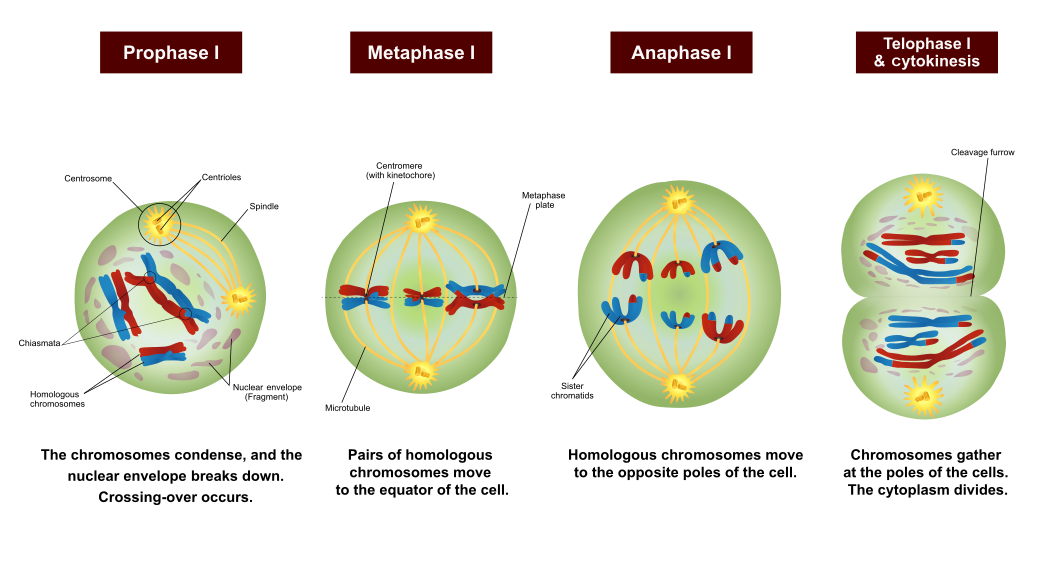
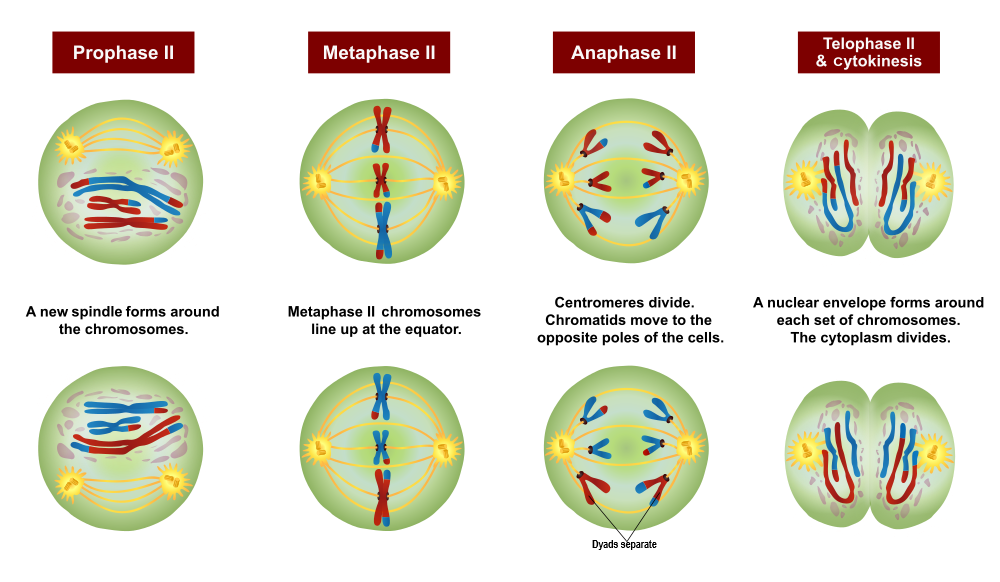
- Anaphase
The stage of mitosis after metaphase and before telophase, when replicated chromosomes are split and the newly-copied chromosomes (daughter chromatids) are moved to opposite poles of the cell.
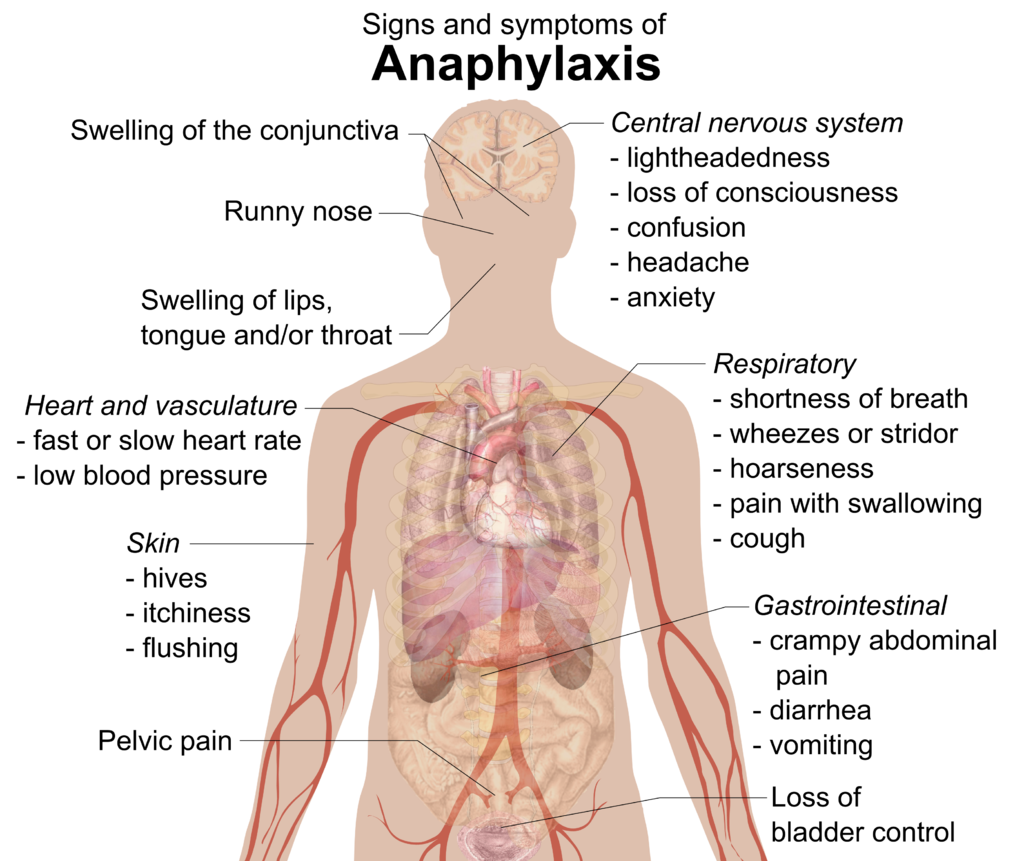
- Anaphylaxis
An acute, potentially life-threatening hypersensitivity reaction, involving the release of mediators from mast cells, basophils and recruited inflammatory cells. Anaphylaxis is defined by a number of signs and symptoms, alone or in combination, which occur within minutes, or up to a few hours, after exposure to a provoking agent. It can be mild, moderate to severe, or severe. Most cases are mild but any anaphylaxis has the potential to become life-threatening.
- Anatomy
The study of the structure of the body.
- Androgen
The general term for a sex hormone predominant in males, such as testosterone.
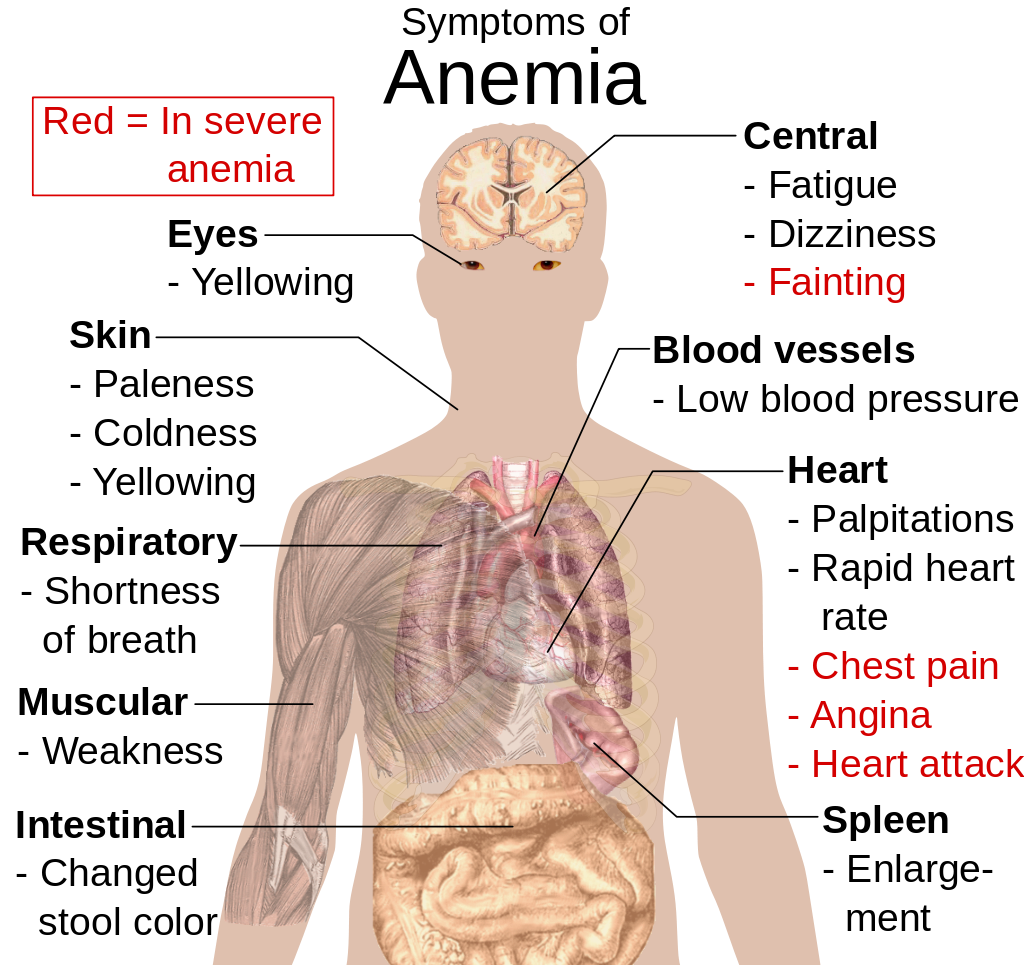
- Anemia
A condition in which you don't have enough healthy red blood cells to carry adequate oxygen to the body's tissues resulting in symptoms including weakness and fatigue.
- Angina
The chest pain or pressure that occurs when heart muscle cells do not receive adequate blood flow and become starved of oxygen.
- Antagonists
A drug that decreases the activity of a particular neurotransmitter.
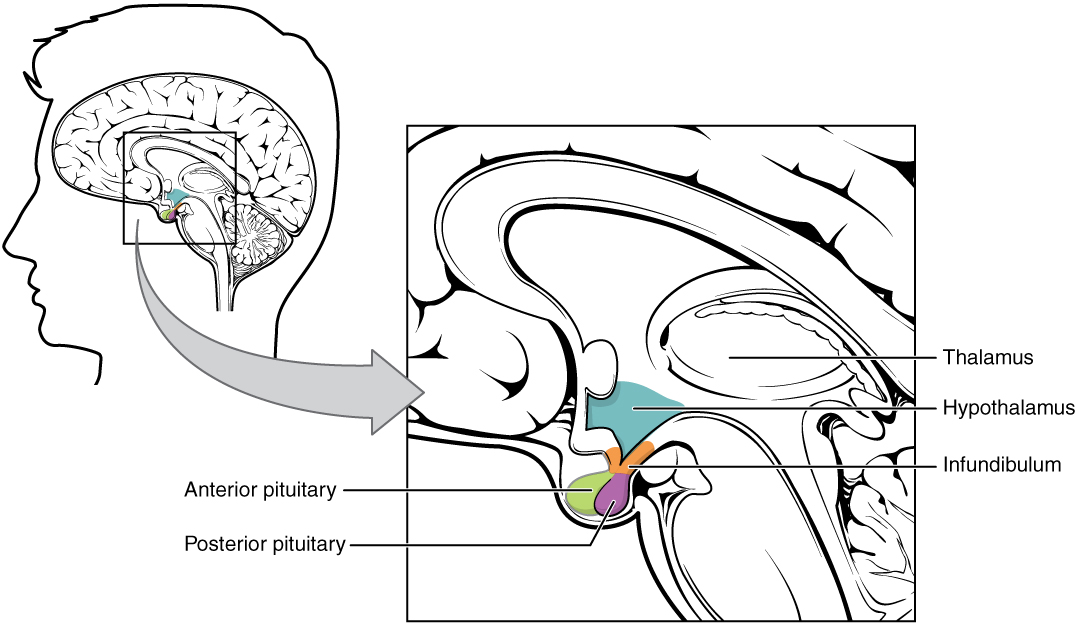
- Anterior pituitary
The front lobe of the pituitary gland that synthesizes and secretes pituitary hormones.
- Anterior pituitary gland
The front lobe of the pituitary gland that synthesizes and secretes pituitary hormones.
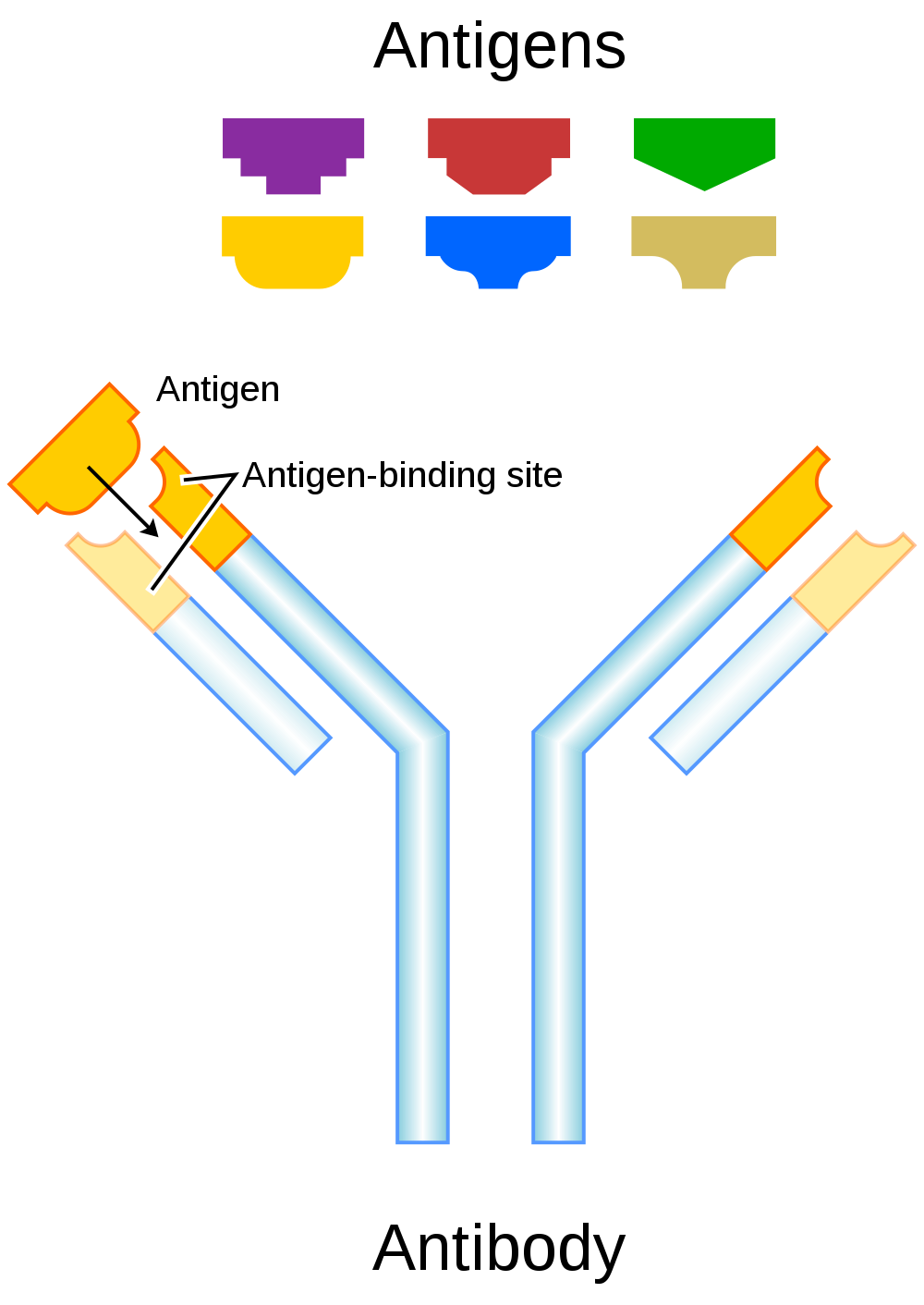
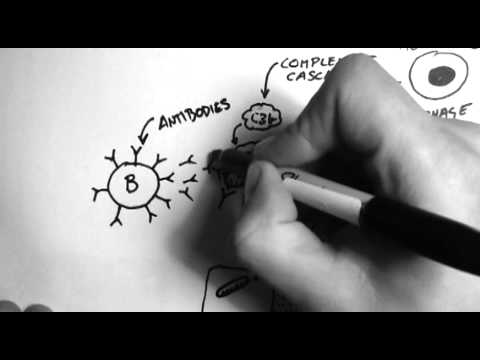
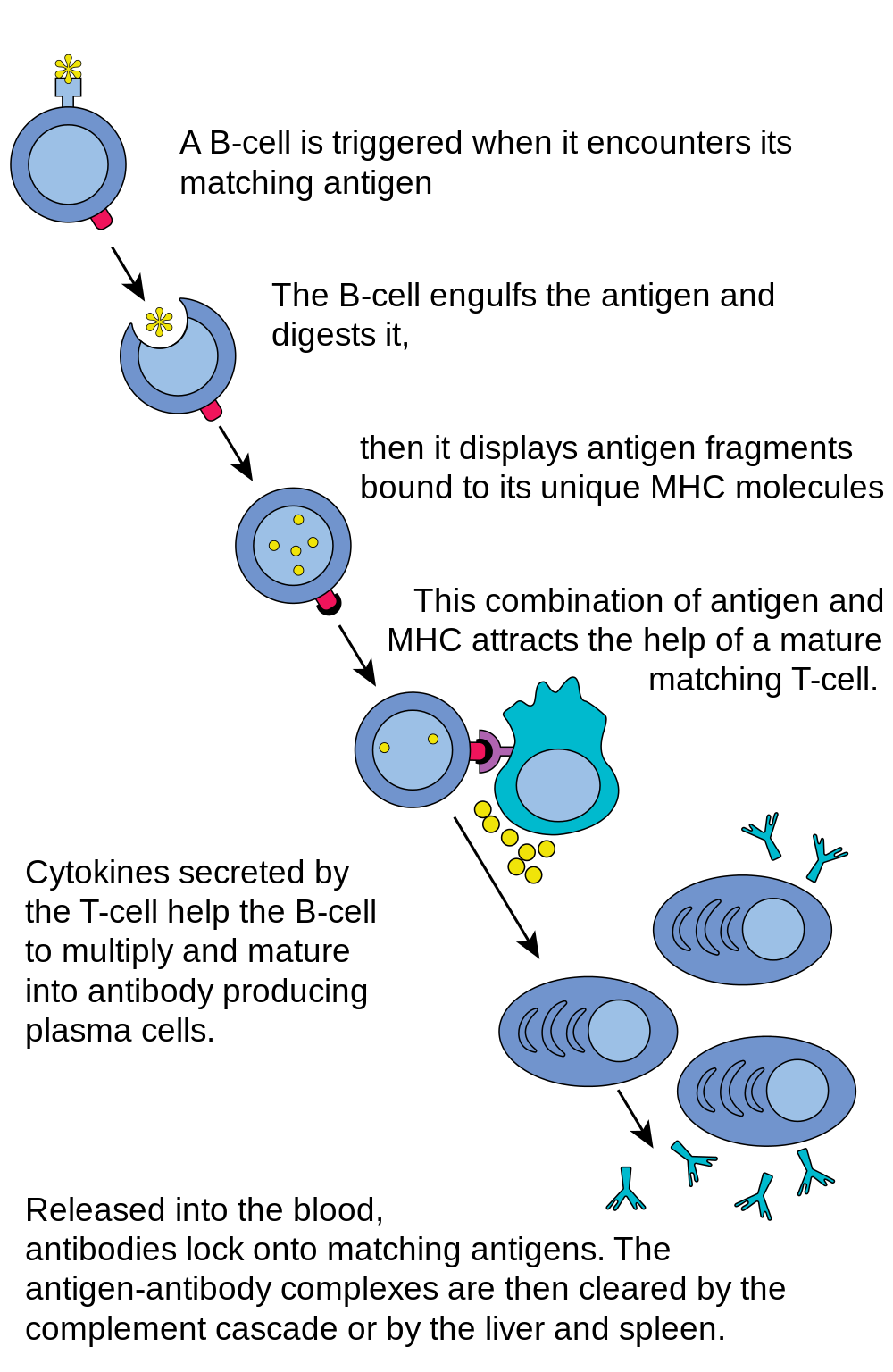
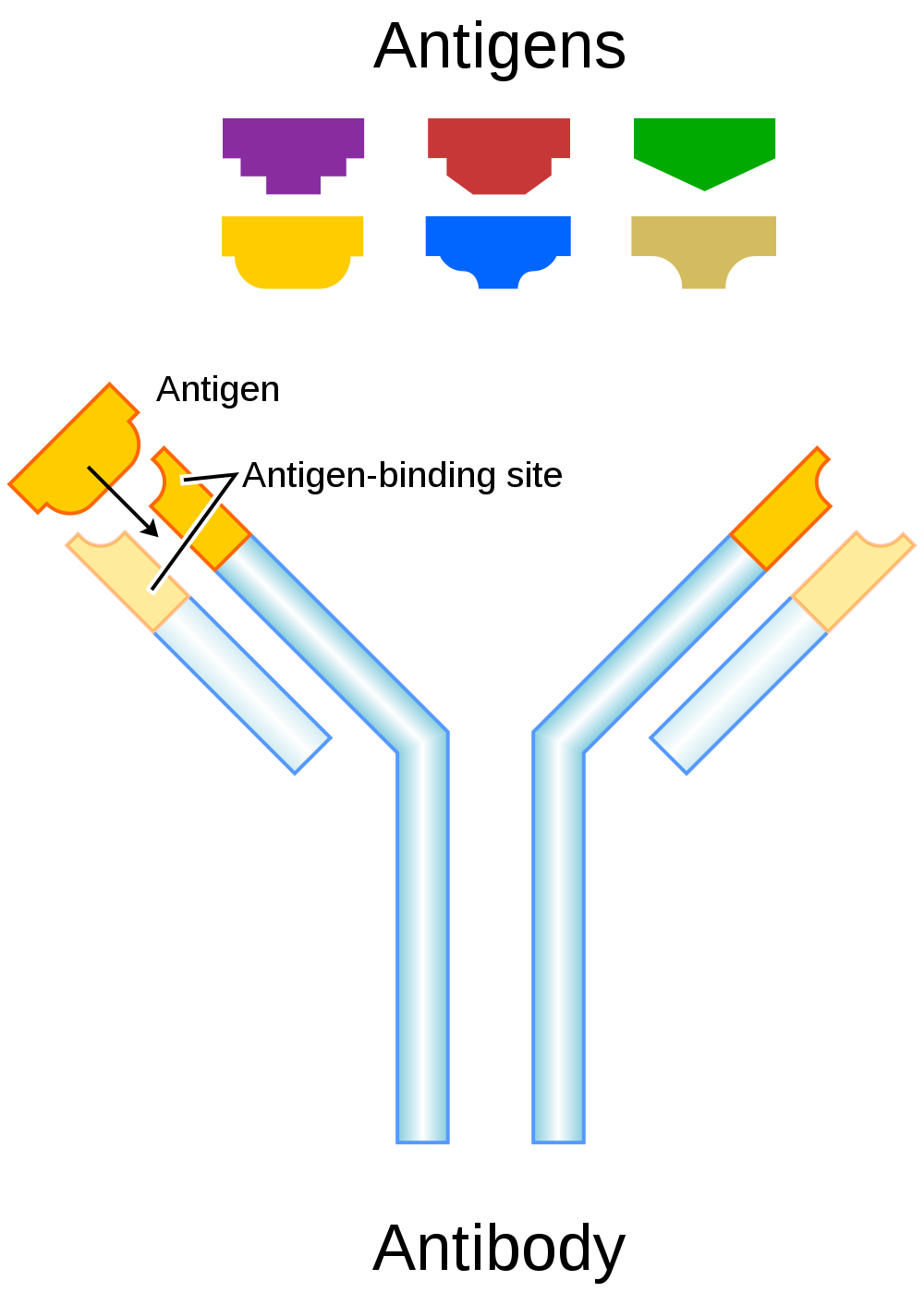
- Antibodies
An antibody, also known as an immunoglobulin, is a large, Y-shaped protein produced mainly by plasma cells that is used by the immune system to neutralize pathogens such as pathogenic bacteria and viruses.
- Anticodon
A three-nucleotide sequence found at one end of a tRNA complementary to that of a corresponding codon in a messenger RNA (mRNA) sequence.
- Antidepressant
Medications used to treat major depressive disorder, some anxiety disorders, some chronic pain conditions, and to help manage some addictions. Common side-effects of antidepressants include dry mouth, weight gain, dizziness, headaches, and sexual dysfunction.
- Antidiuretic hormone
A hormone made by the hypothalamus in the brain and stored in the posterior pituitary gland. It tells your kidneys how much water to conserve. ADH constantly regulates and balances the amount of water in your blood. Higher water concentration increases the volume and pressure of your blood.
- Antidiuretic hormone (ADH)
also called vasopressin. A hormone made by the hypothalamus in the brain and stored in the posterior pituitary gland. It tells your kidneys how much water to conserve. ADH constantly regulates and balances the amount of water in your blood.
- Antigen
Molecules on the surface of cells or viruses that the immune system identifies as either self (produced by your own body) or non-self (not produced by your own body).
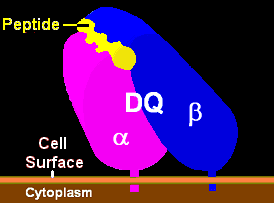
- Antigen-presenting cell
A type of immune cell that enables a T lymphocyte (T cell) to recognize an antigen and mount an immune response against the antigen. Antigen-presenting cells (APCs) include macrophages, dendritic cells, and B lymphocytes (B cells).
- Antihistamine
Drugs that combat the histamine released during an allergic reaction by blocking the action of the histamine on the tissue.
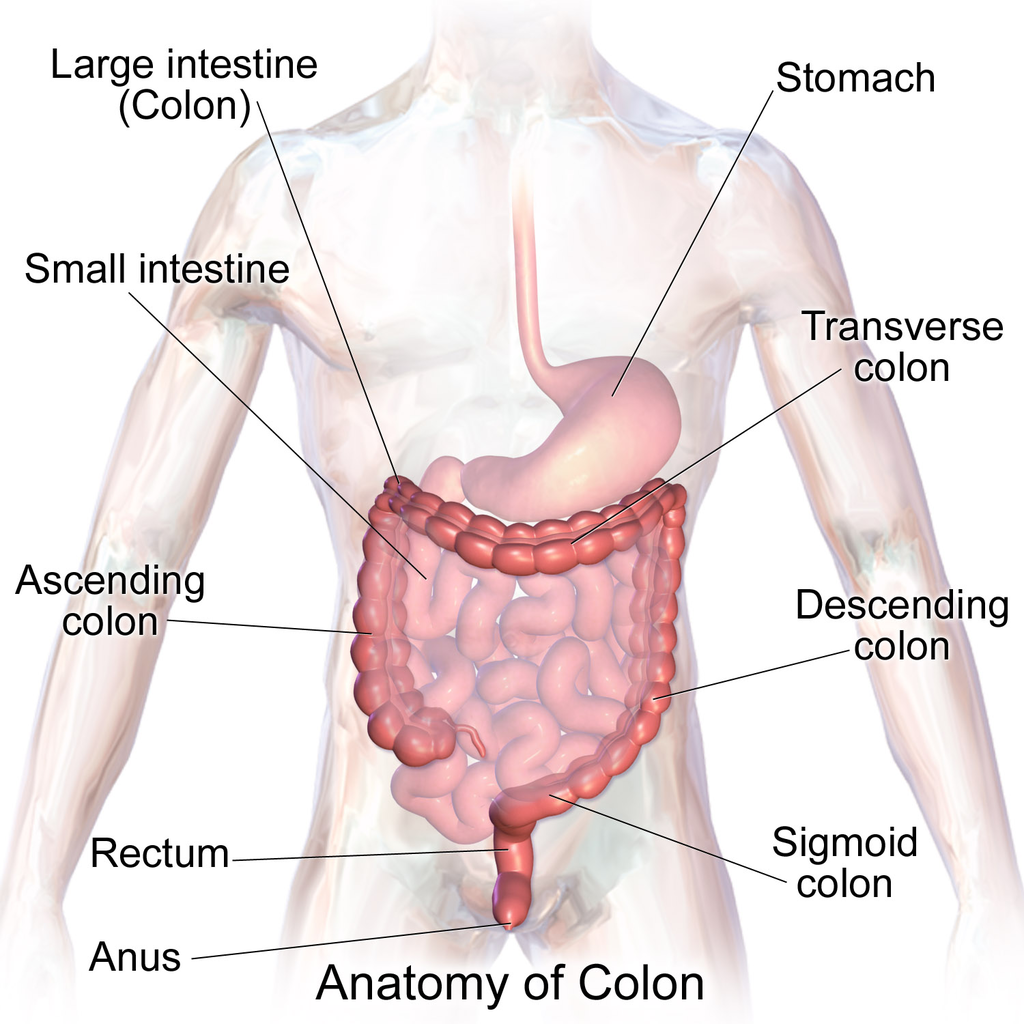
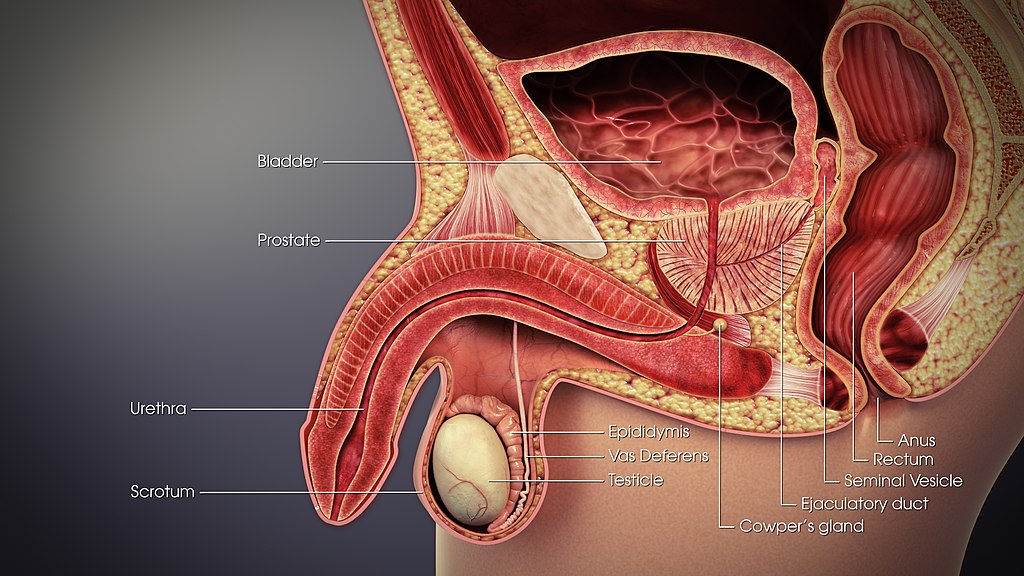
- Anus
The final part of the large intestine with an opening to the outside for feces to pass through.
- Anxiolytics
Type of psychoactive drug that has a tranquilizing effect and inhibits anxiety.
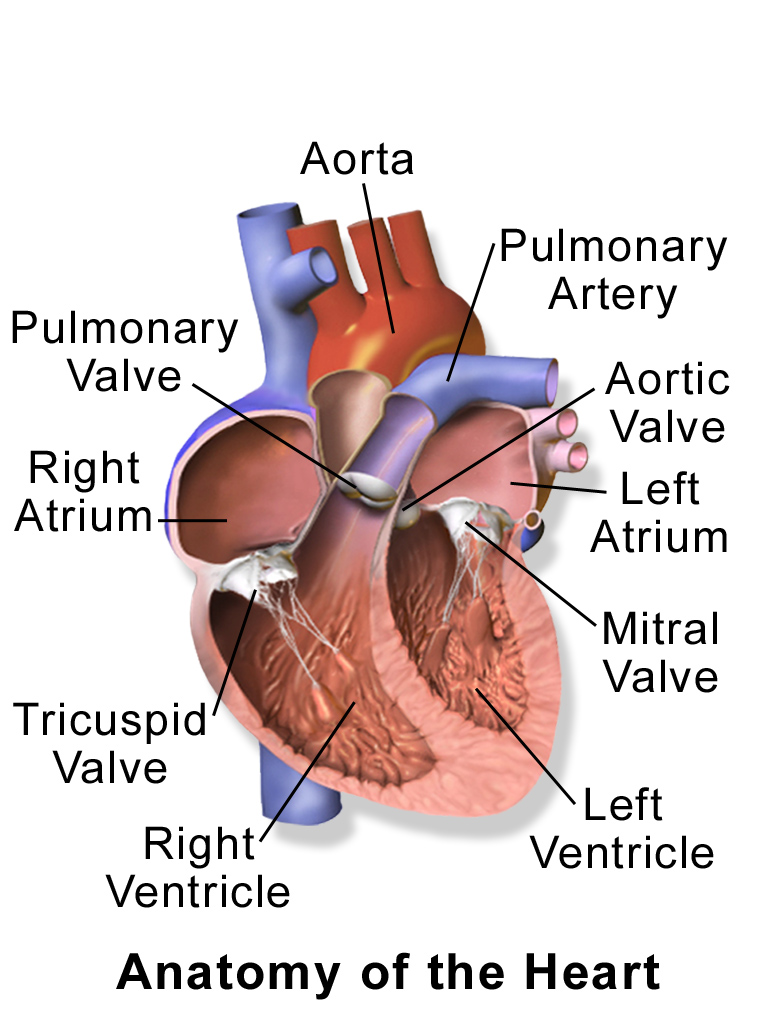
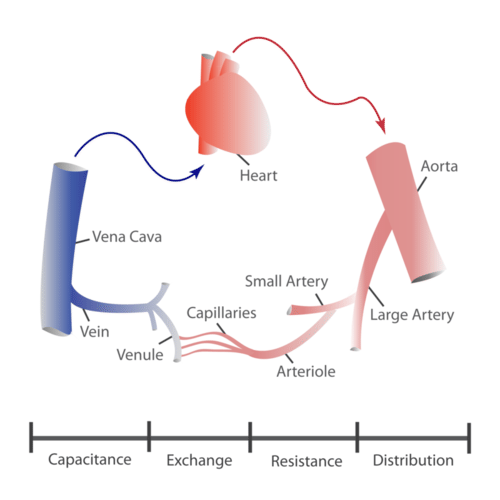
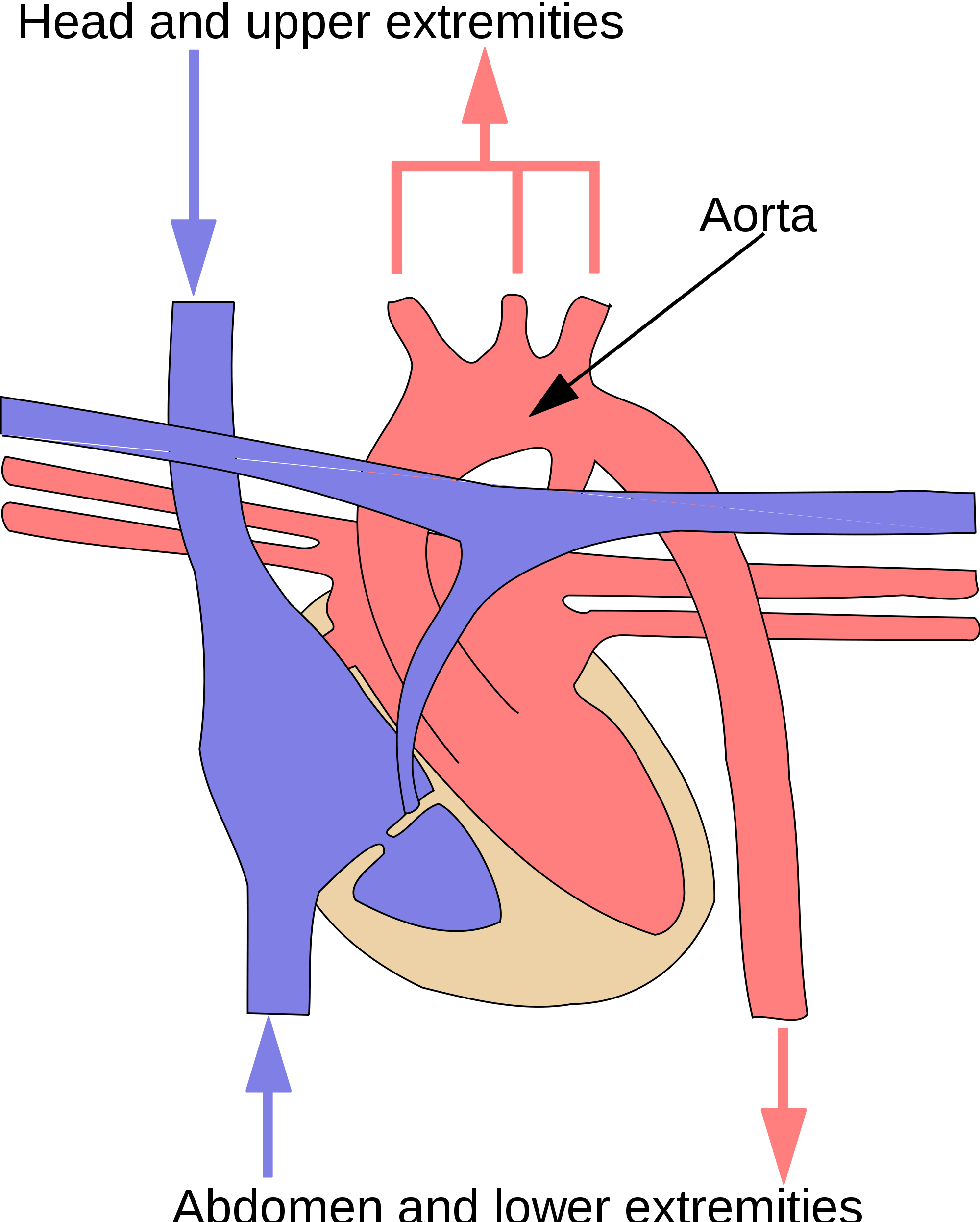
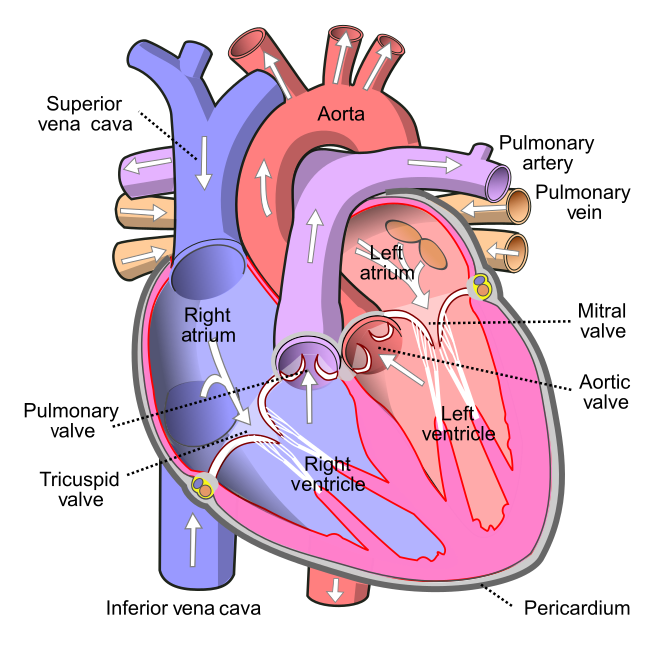
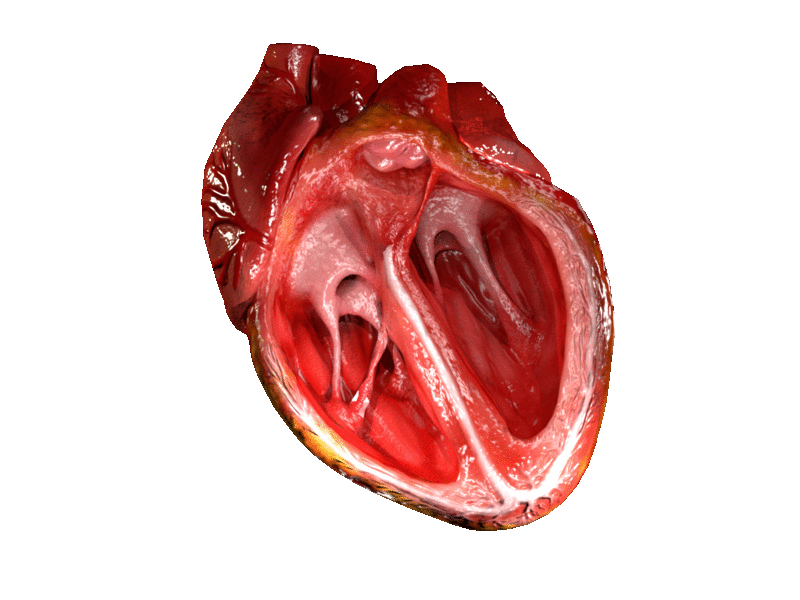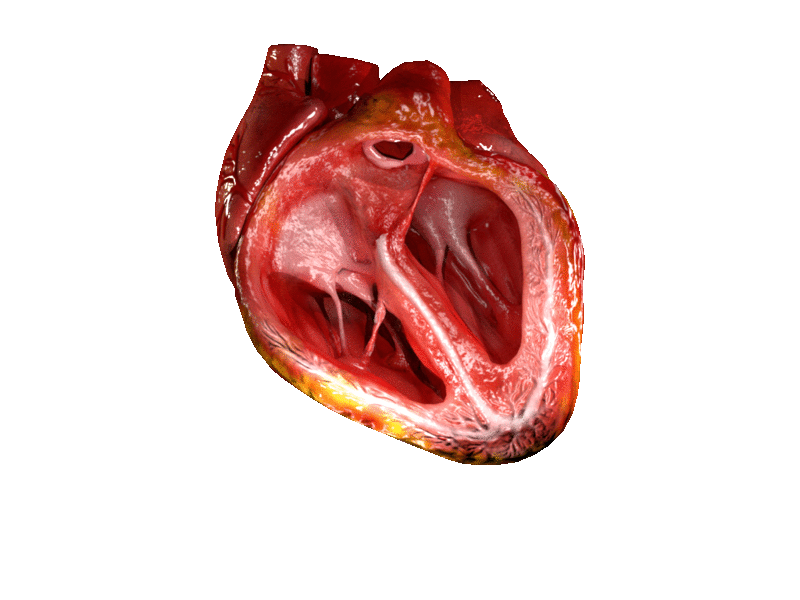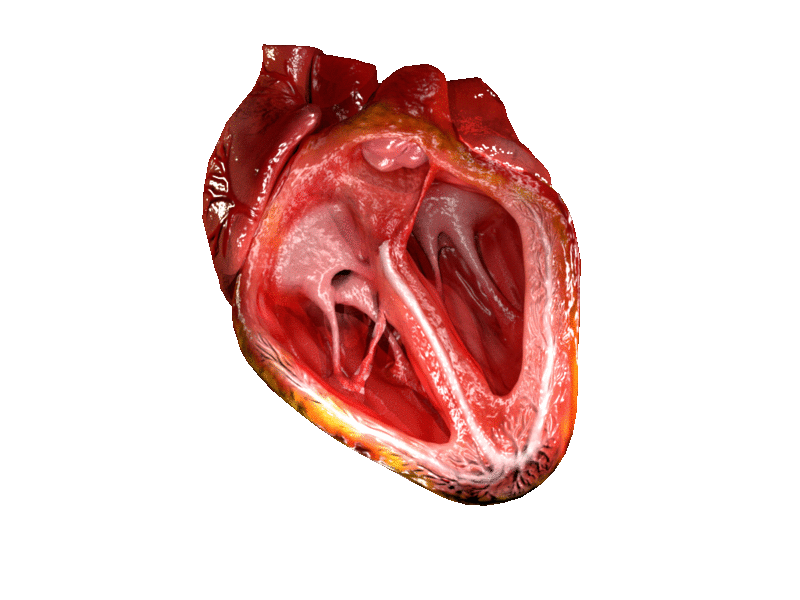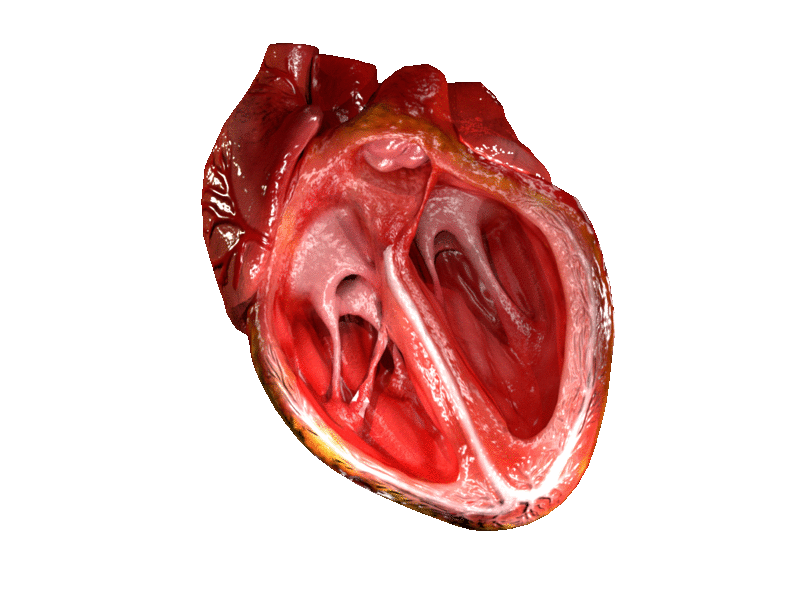
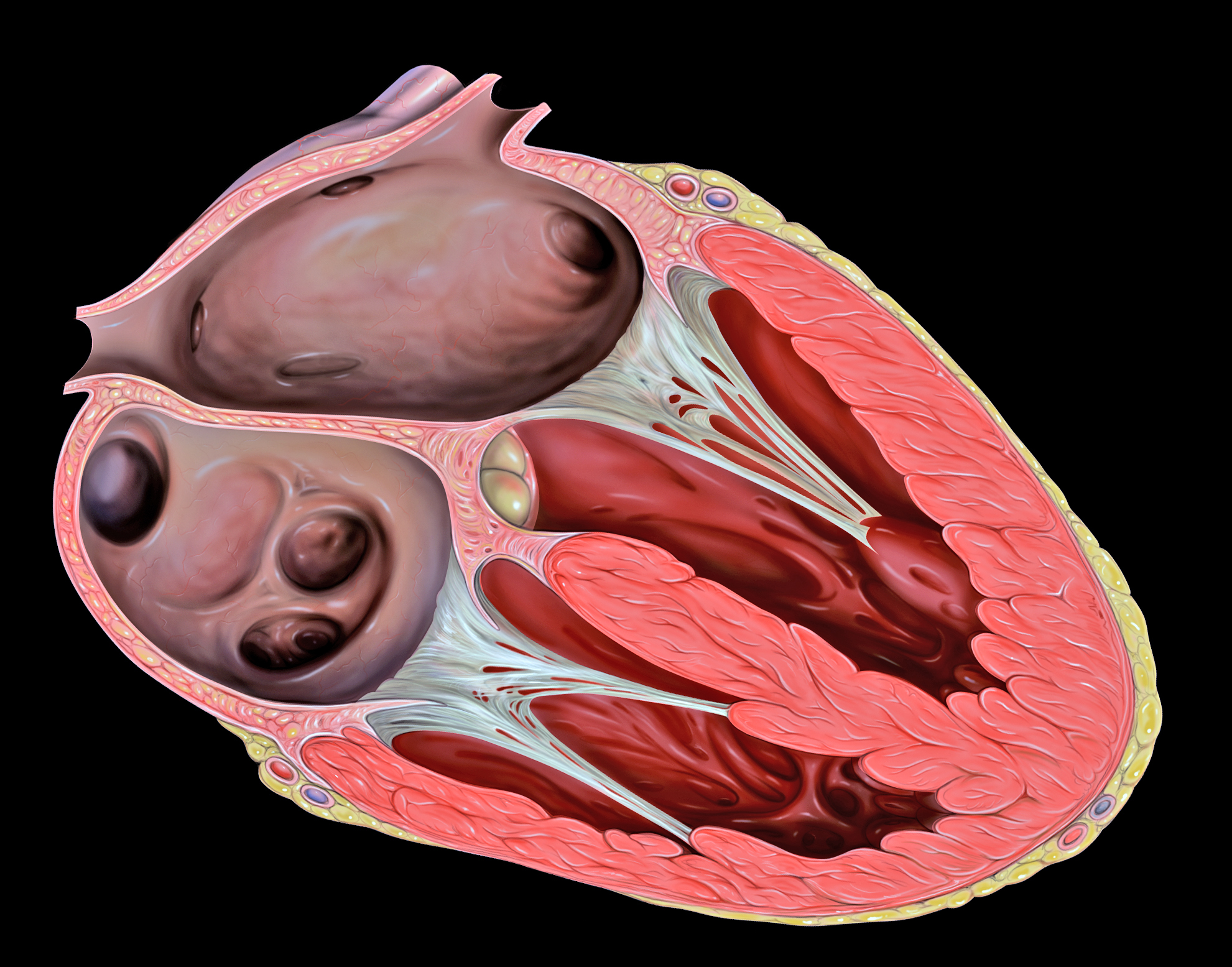
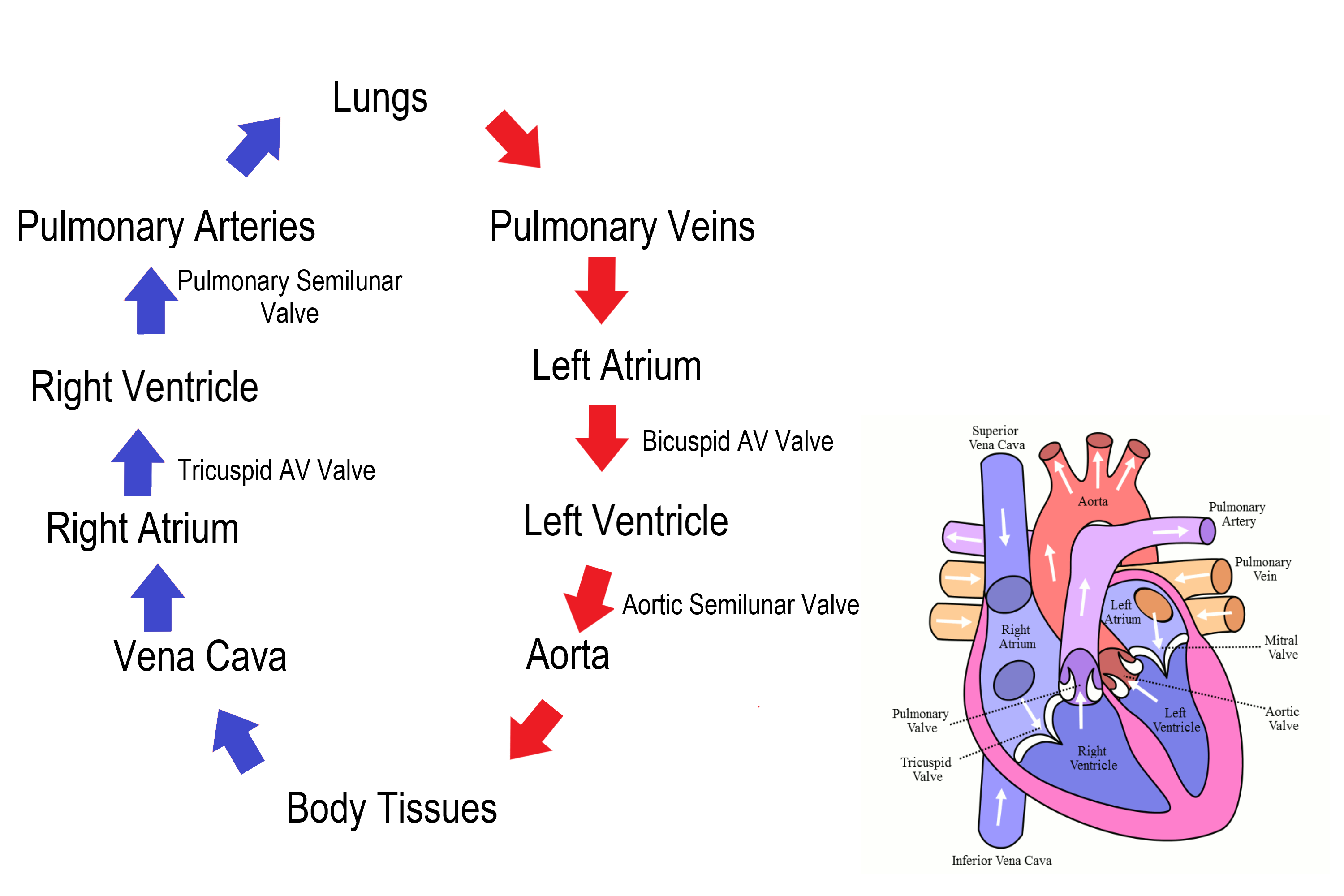
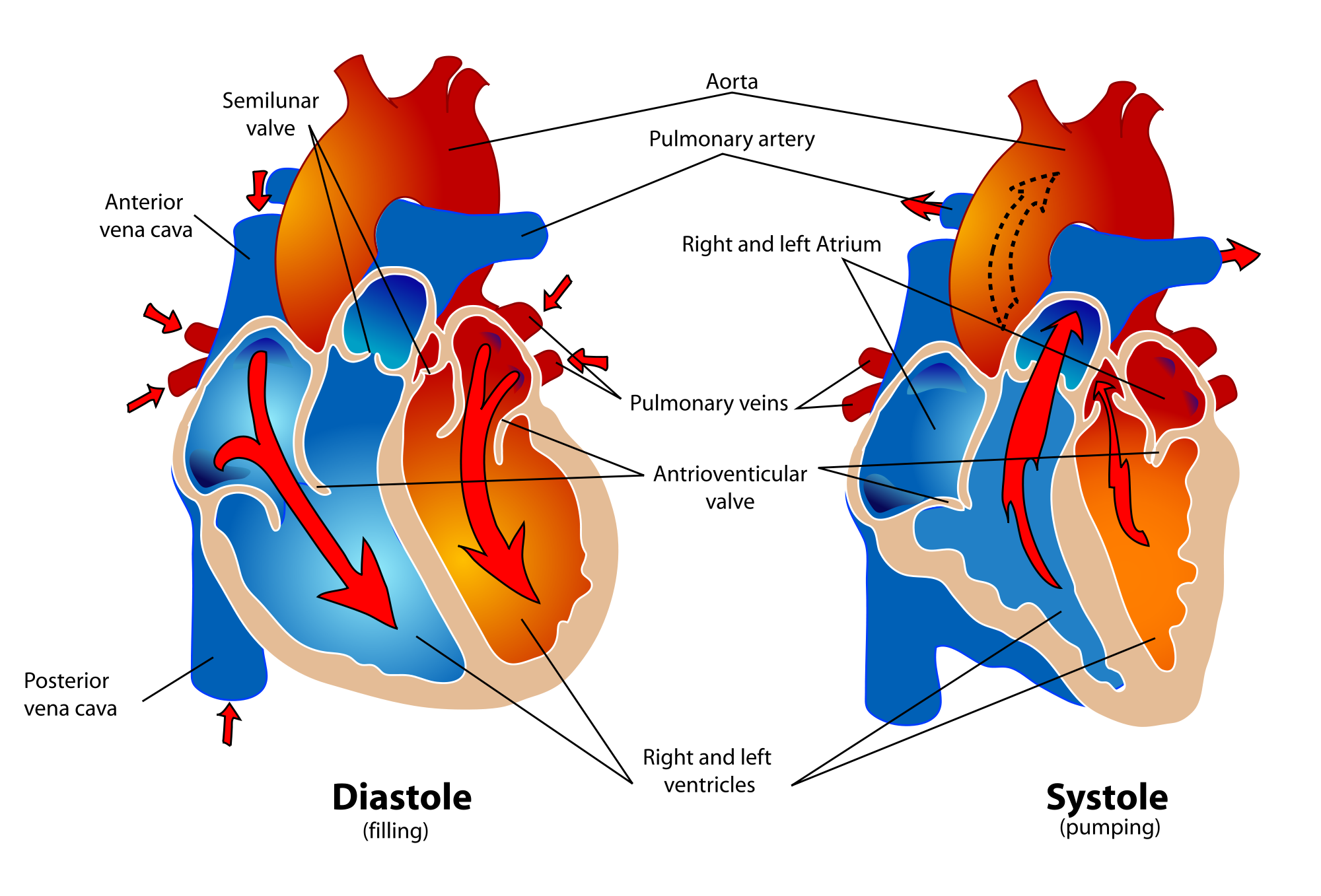
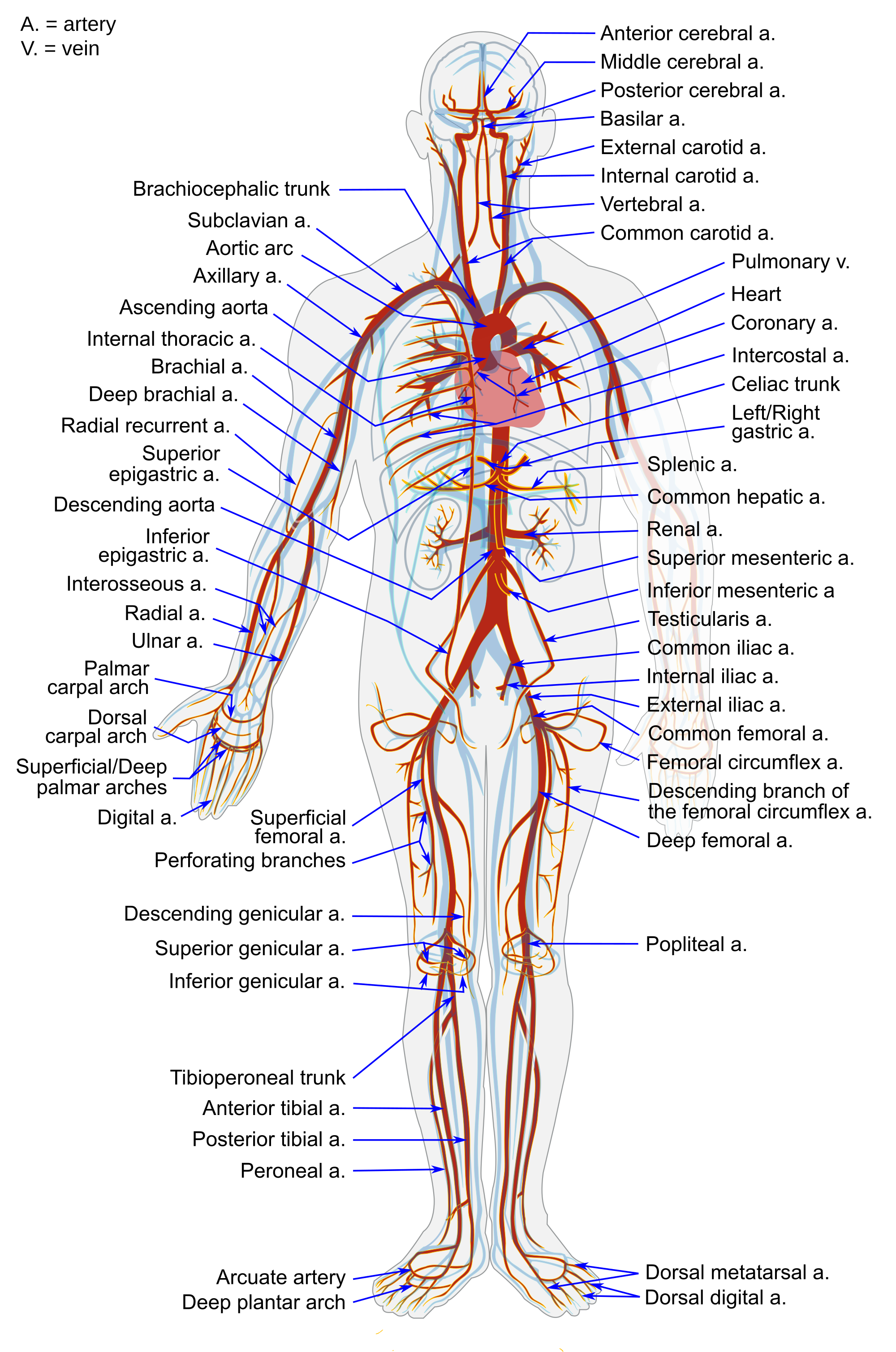
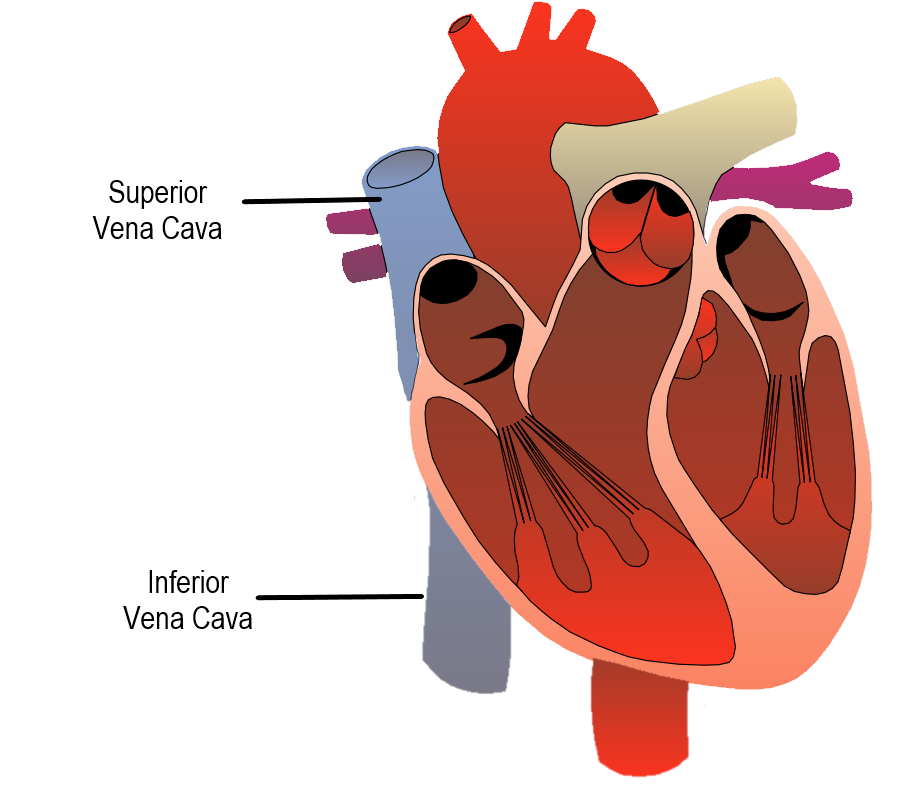
- Aorta
The main artery of the body, supplying oxygenated blood to the circulatory system. In humans it passes over the heart from the left ventricle and runs down in front of the backbone.
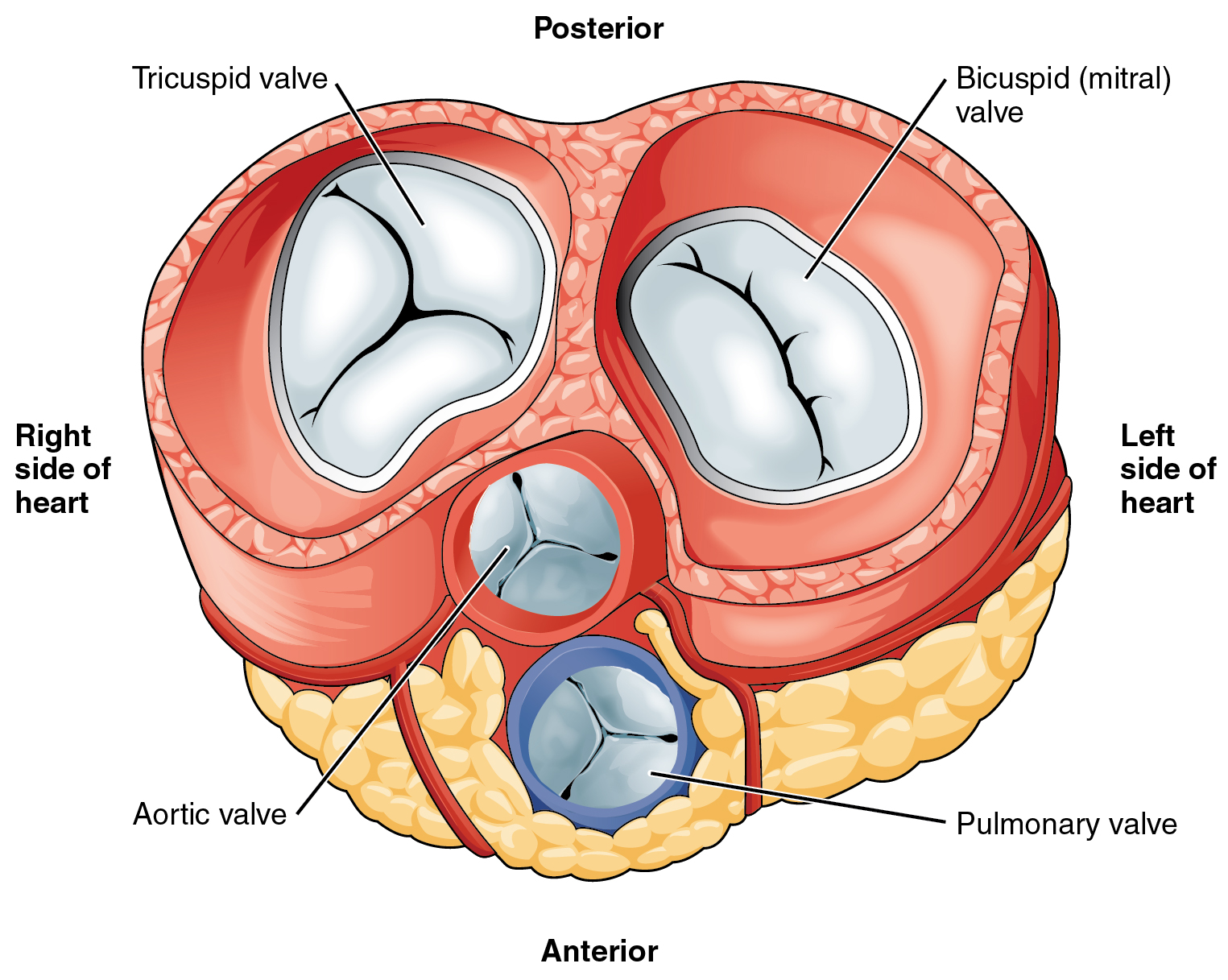
- Aortic semilunar valve
A semilunar valve in the hearth that separates the left ventricle and the aorta; preventing backflow of blood.
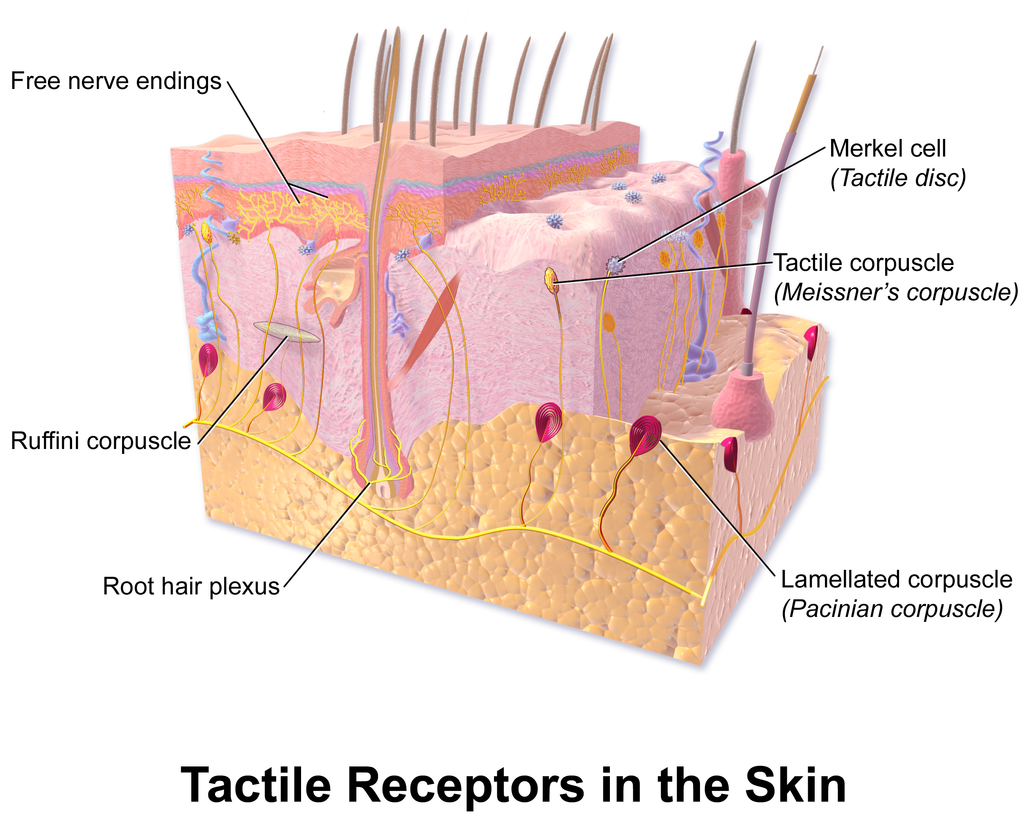
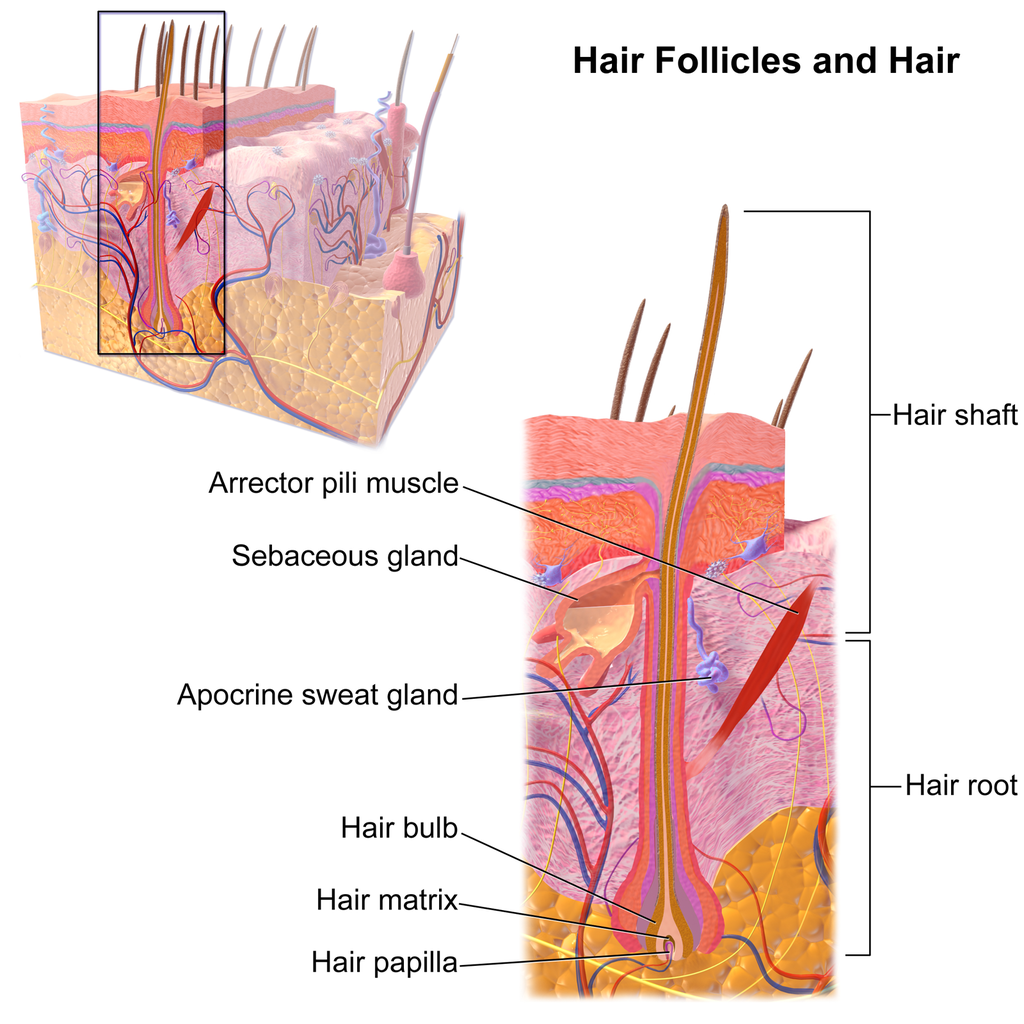
- Apocrine sweat gland
Sweat glands that secrete their products into a hair follicle. Present only in certain places in the human body including the armpits, nipples, ear canal, eyelids, and parts of the external genitalia.
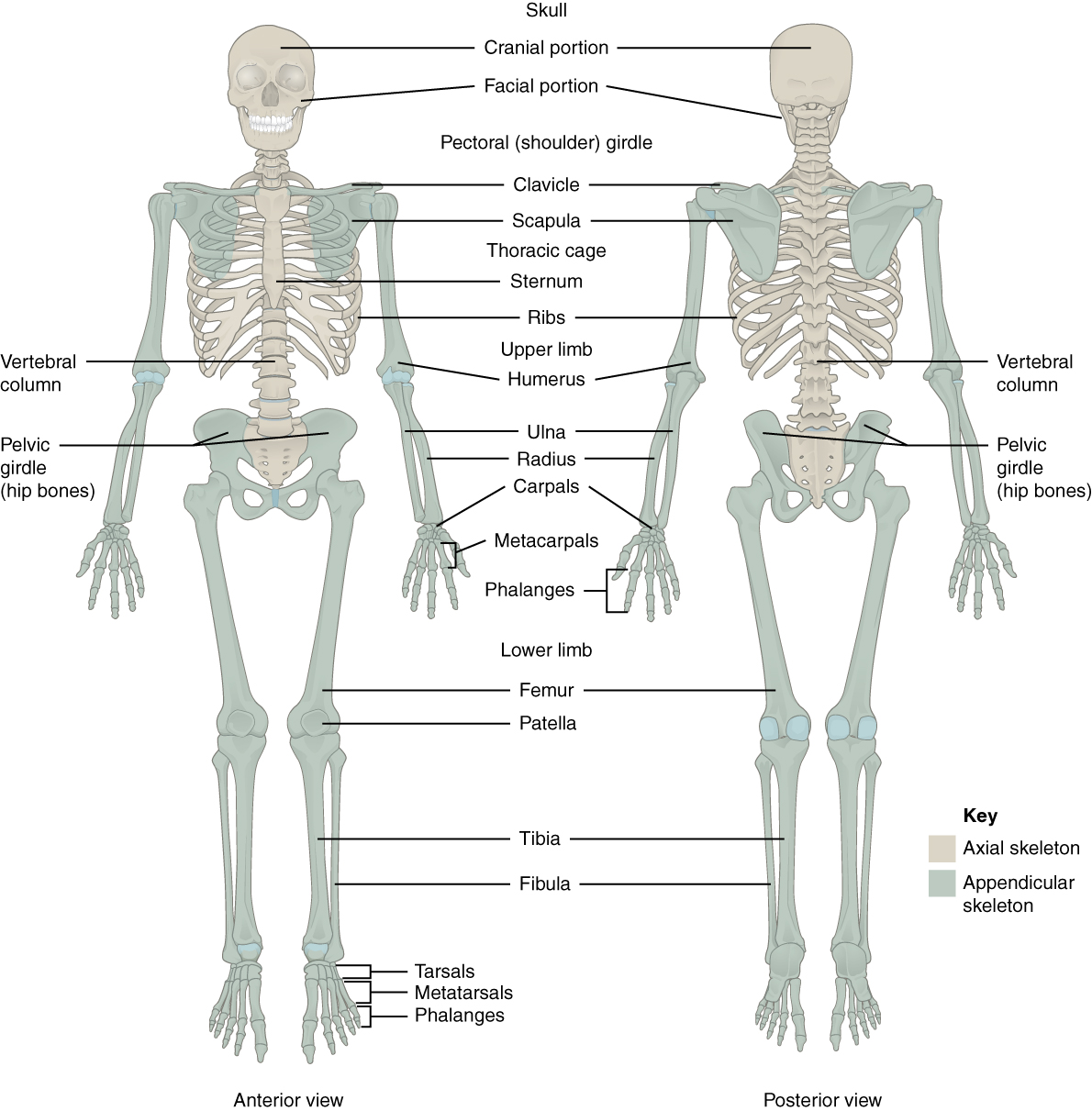
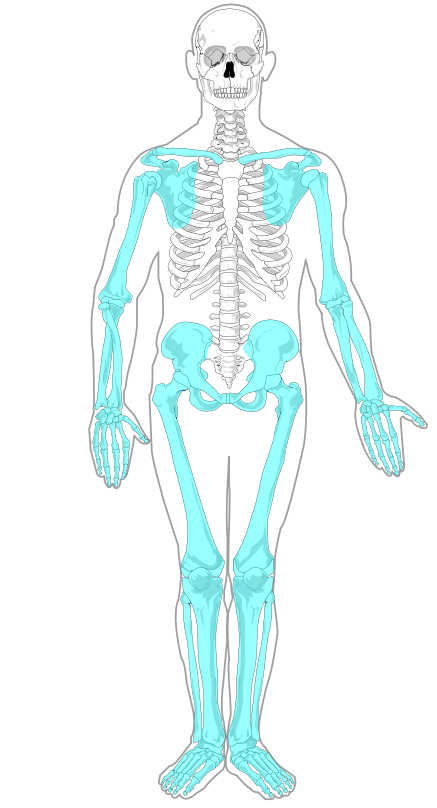
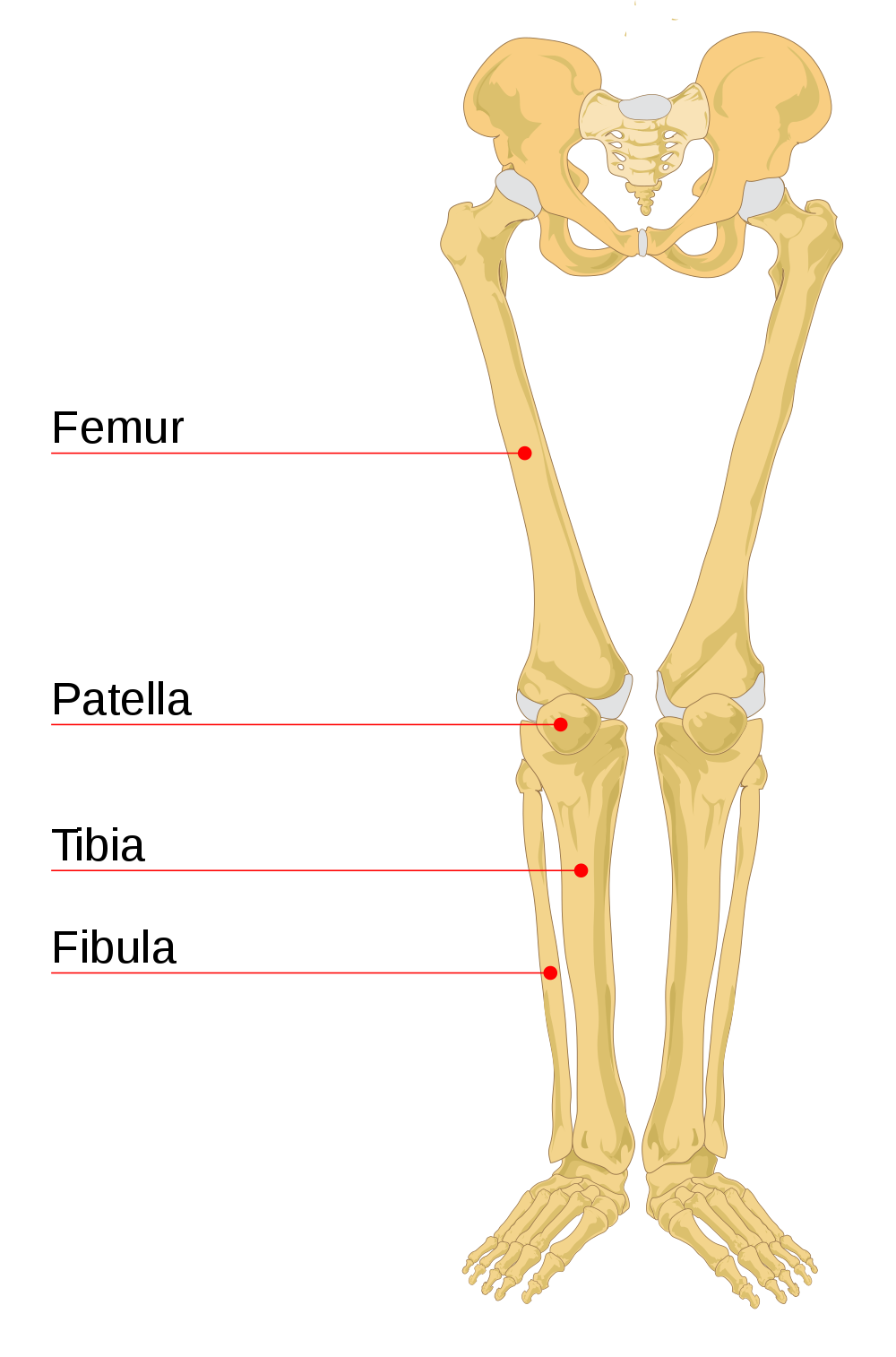
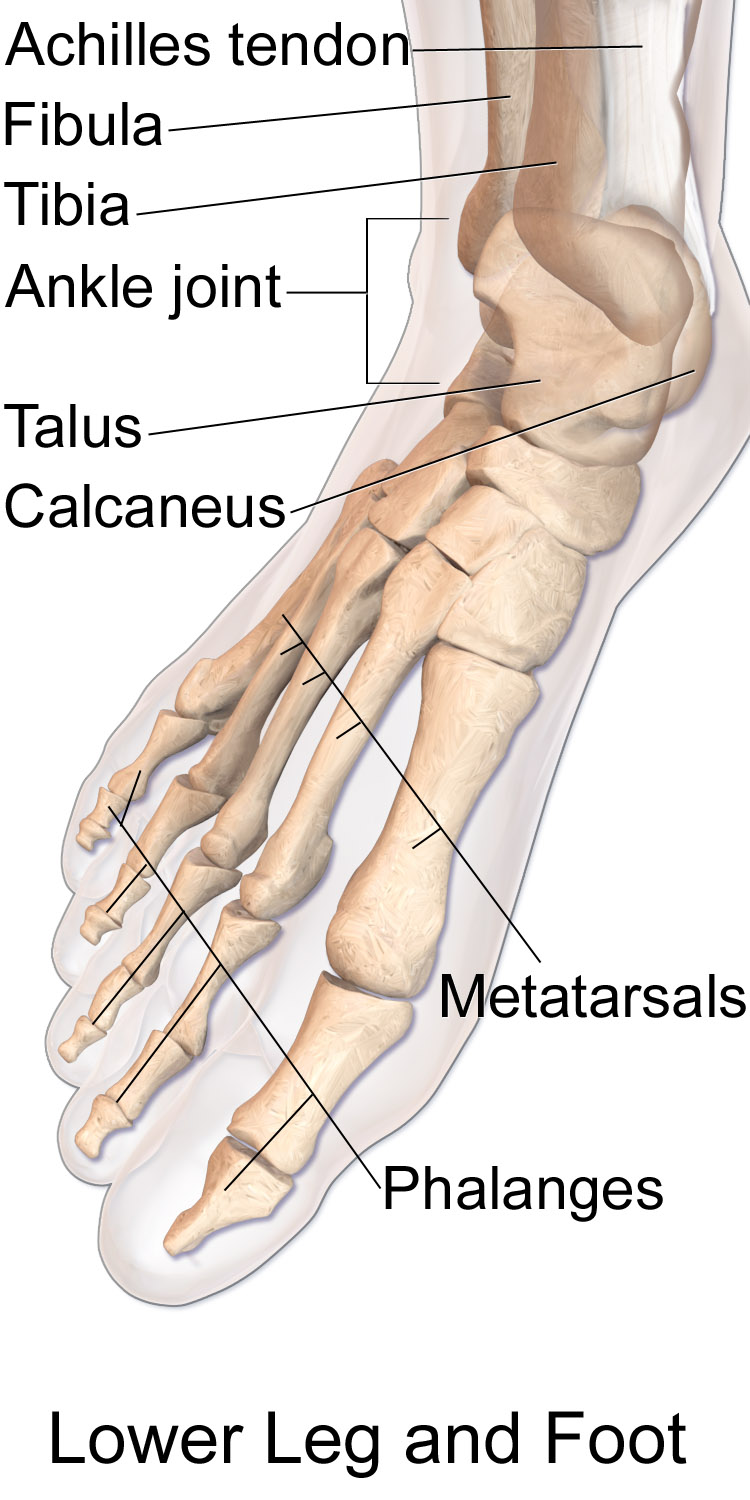
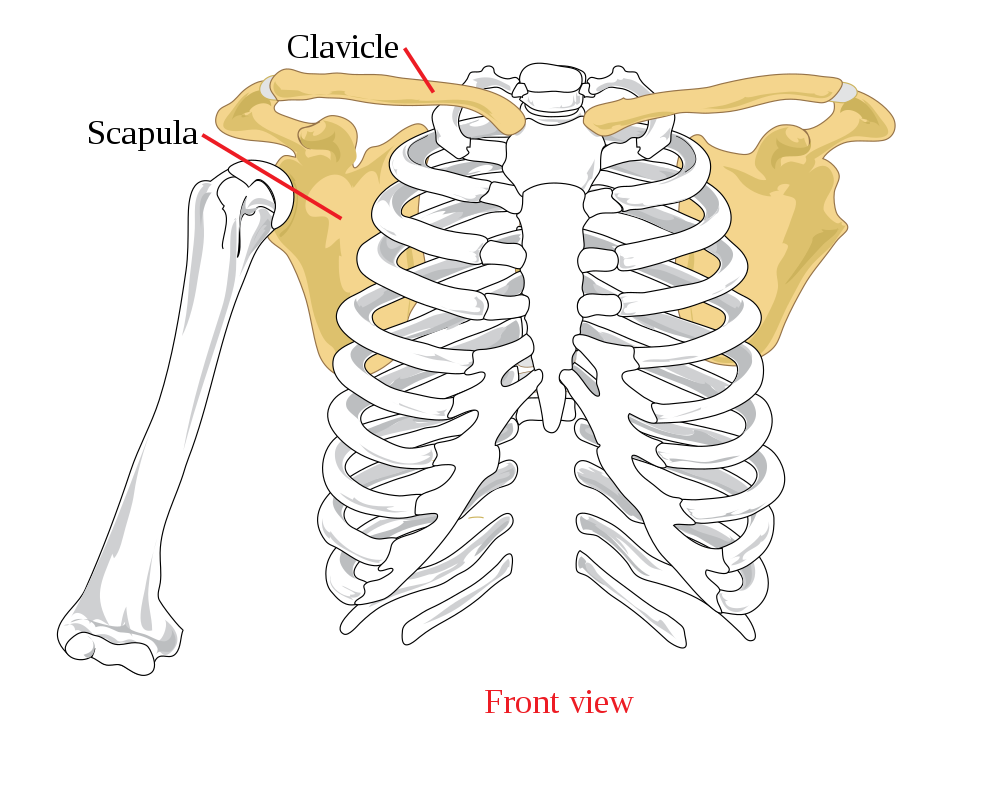
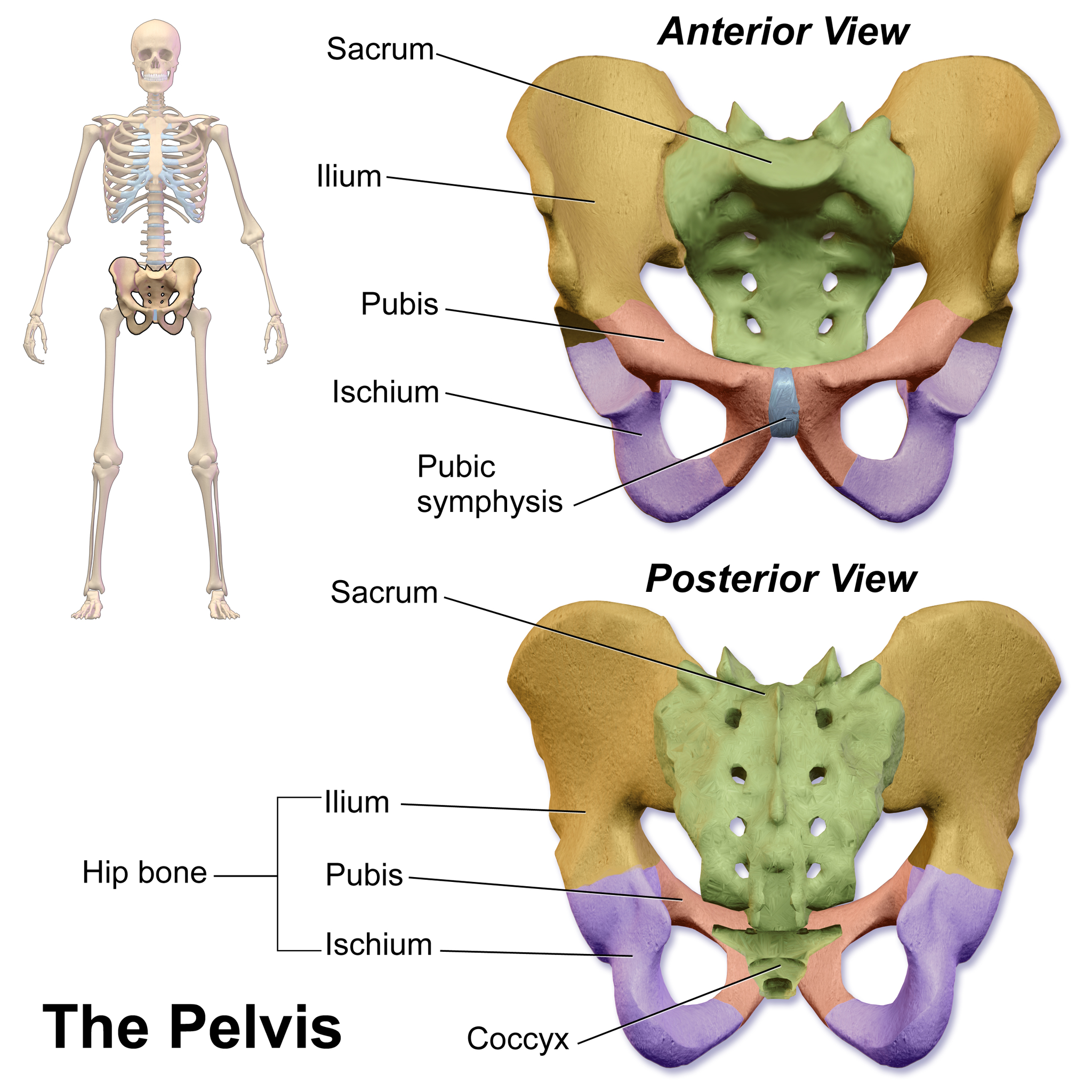
- Appendicular skeleton
The bones of the upper and lower limbs, shoulder girdle, and pelvic girdle.
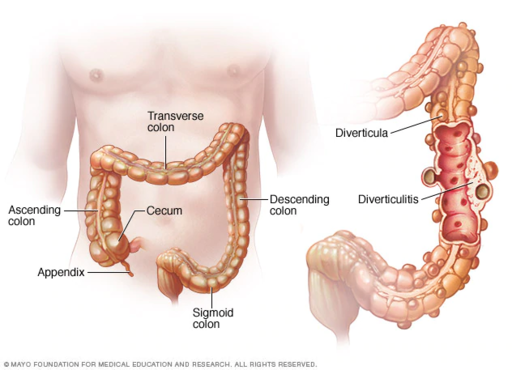
- Appendix
A tube-shaped sac attached to and opening into the lower end of the large intestine in humans and some other mammals. Some of the functions of the appendix include maintaining gut flora and immune and lymphatic function.
- Aqueous humor
A transparent watery fluid similar to plasma, but containing low protein concentrations. It is secreted from the ciliary epithelium, a structure supporting the lens.
- Arrector pili
Small muscles attached to hair follicles in mammals. Contraction of these muscles causes the hairs to stand on end, known colloquially as goose bumps.
- Arrhythmias
A condition in which the heart beats with an irregular or abnormal rhythm.
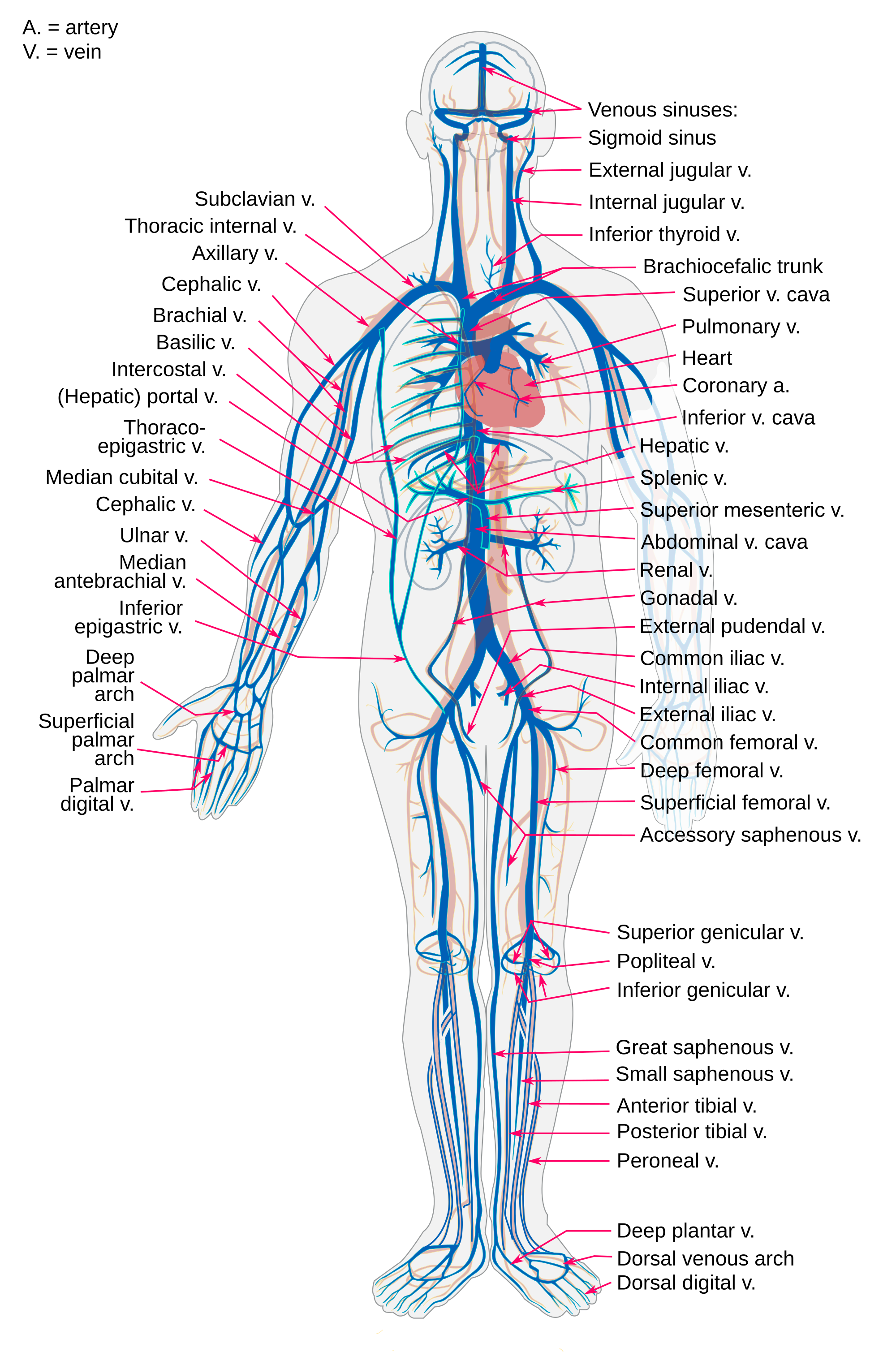
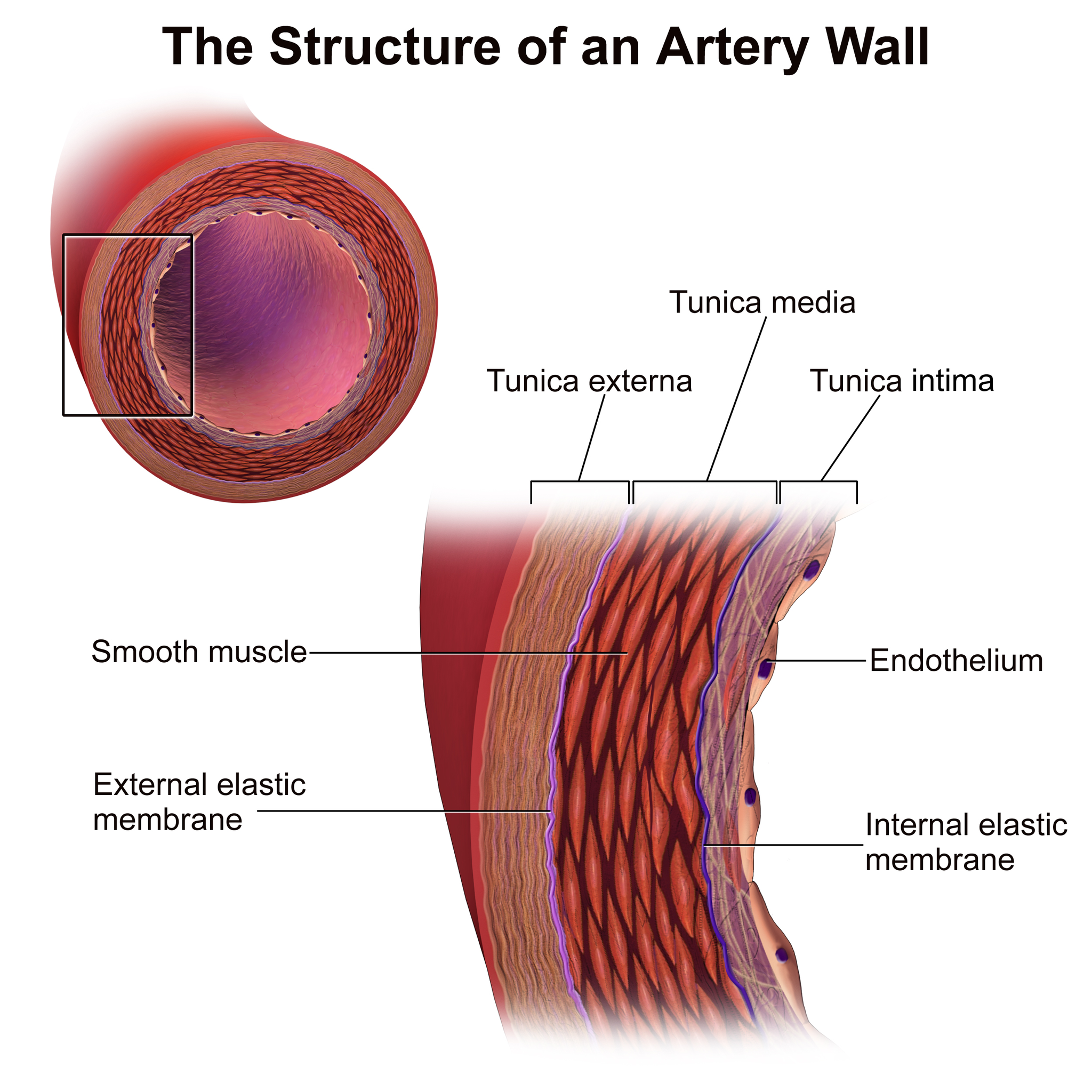
- Artery
A type of blood vessel that carries blood away from the heart and toward the lungs or body.
- Artificial selection
The identification by humans of desirable traits in plants and animals, and the steps taken to enhance and perpetuate those traits in future generations.
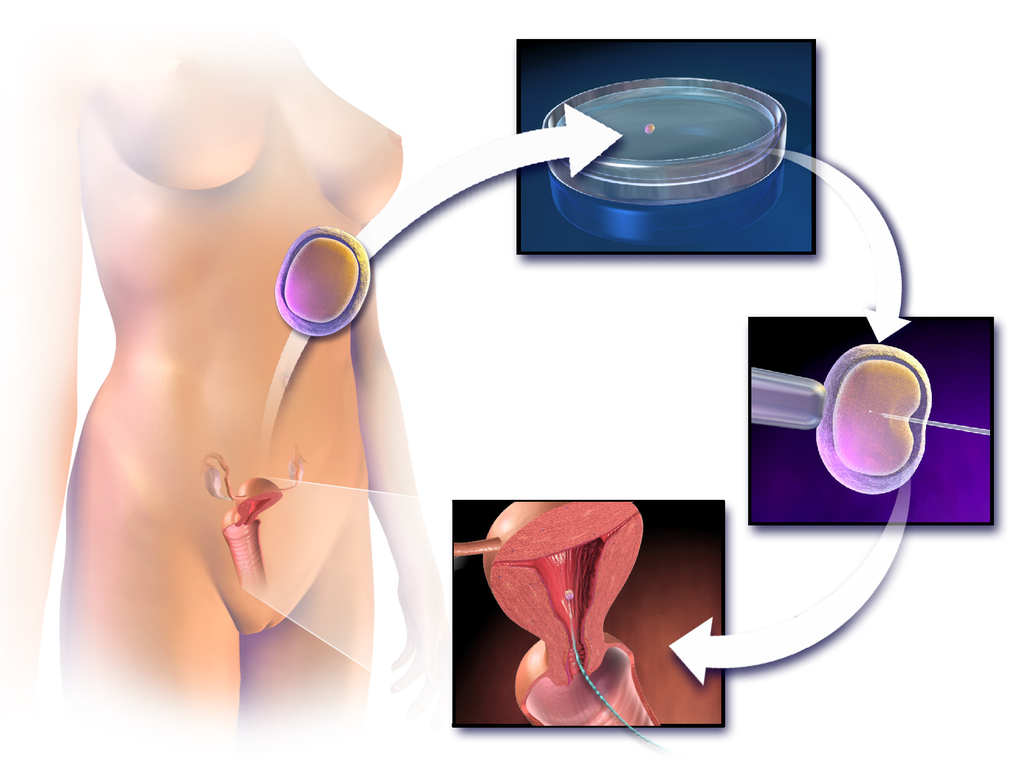
- Assisted reproductive technology (ART)
A collection of medical procedures in which eggs and sperm are removed from an infertile couple and manipulated in ways that increase the chances of fertilization occurring, such as in-vitro fertilization
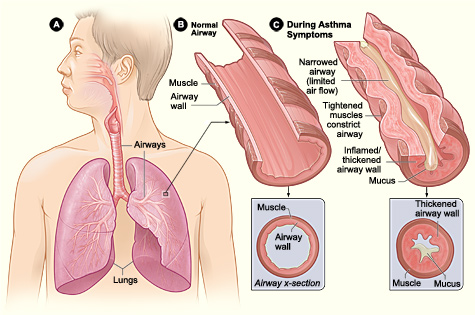
- Asthma
Chronic inflammatory disease of the respiratory system in which airways periodically become inflamed, causing swelling and narrowing of the airways, which makes breathing difficult.
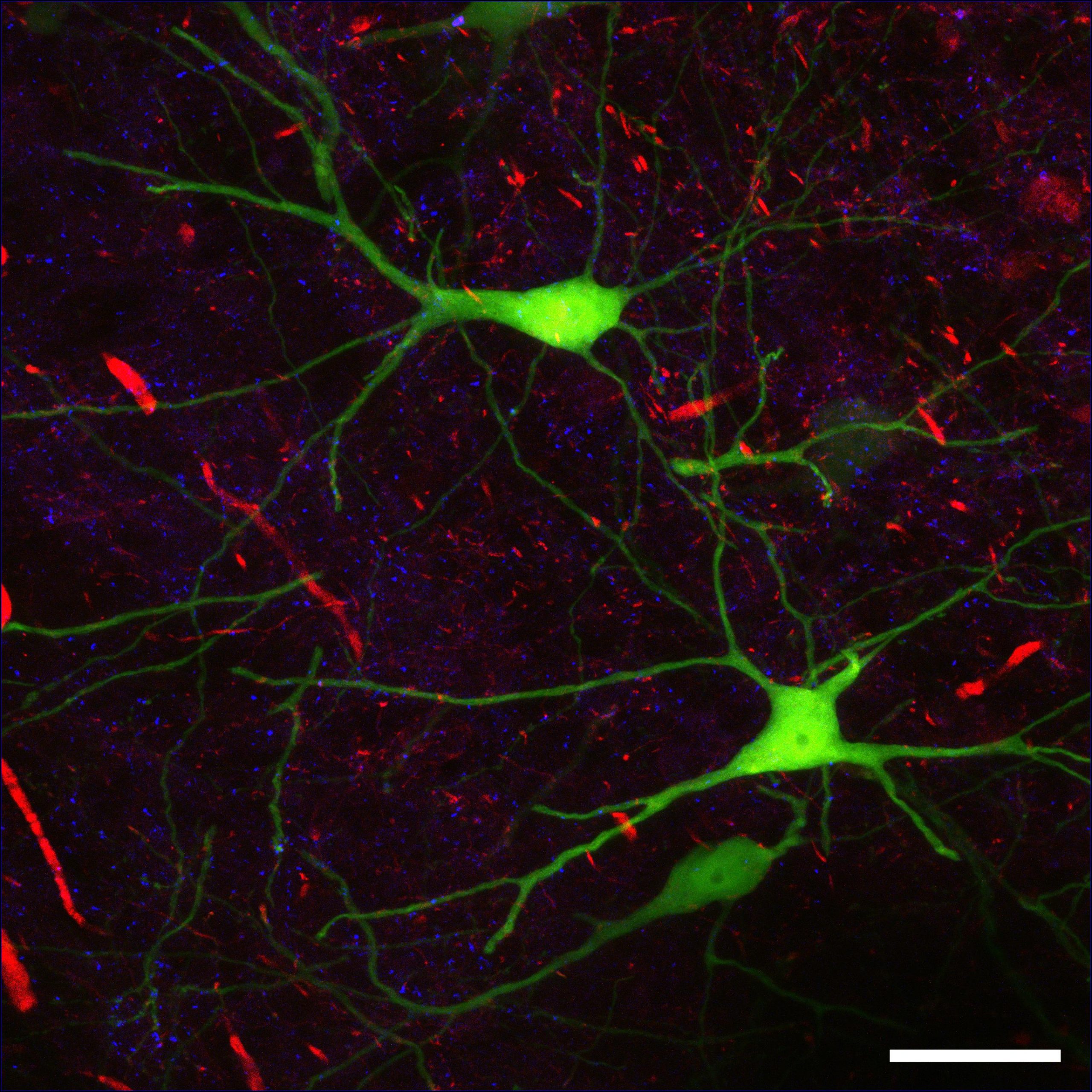
- Astrocytes
Star-shaped neuroglia that have a number of functions, including support of the blood-brain barrier, provision of nutrients to neurons, repair to nervous tissue following injury, and facilitation of neurotransmission.
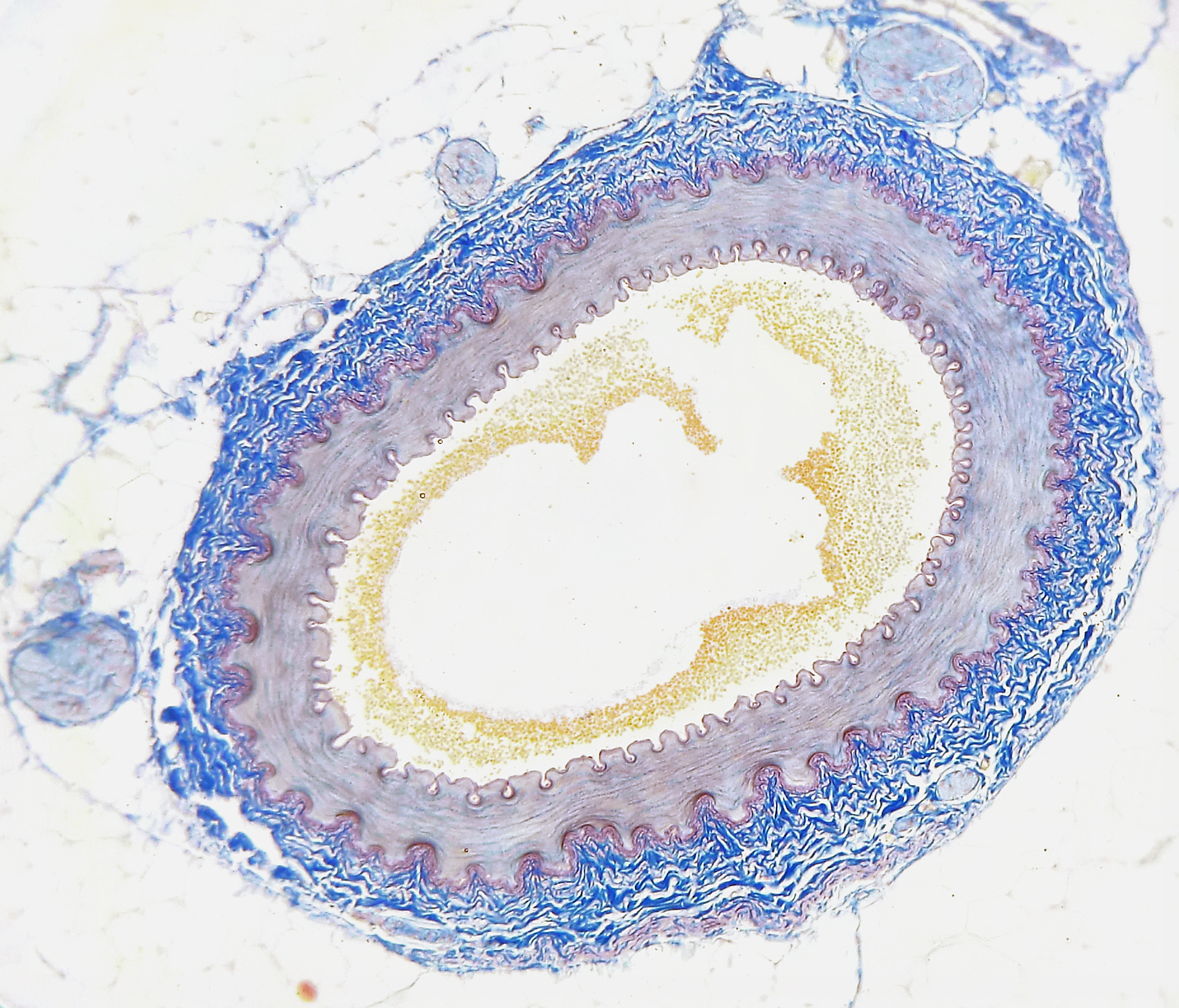
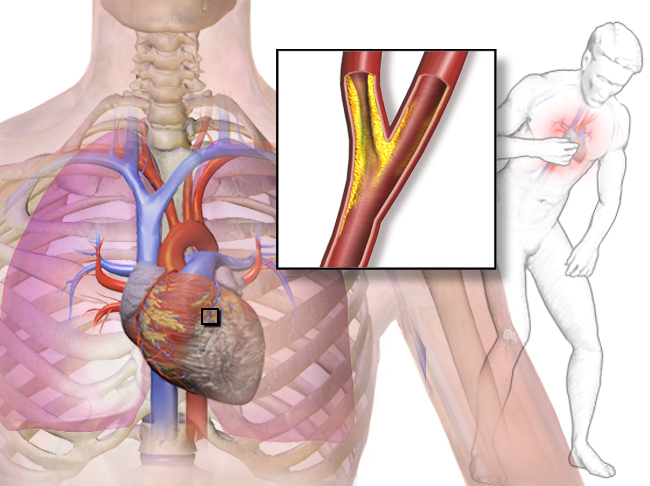
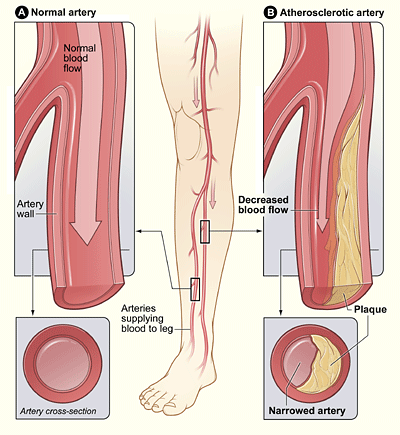
- Atherosclerosis
A condition in which plaque builds up inside arteries, eventually causing the lumen inside to narrow and the arterial walls to stiffen.
- Atom
The smallest particle of an element that still has the properties of that element.
- Atomic nucleus
A small dense region in the center of an atom containing protons and neutrons.
- ATP
A complex organic chemical that provides energy to drive many processes in living cells, e.g. muscle contraction, nerve impulse propagation, and chemical synthesis. Found in all forms of life, ATP is often referred to as the "molecular unit of currency" of intracellular energy transfer.
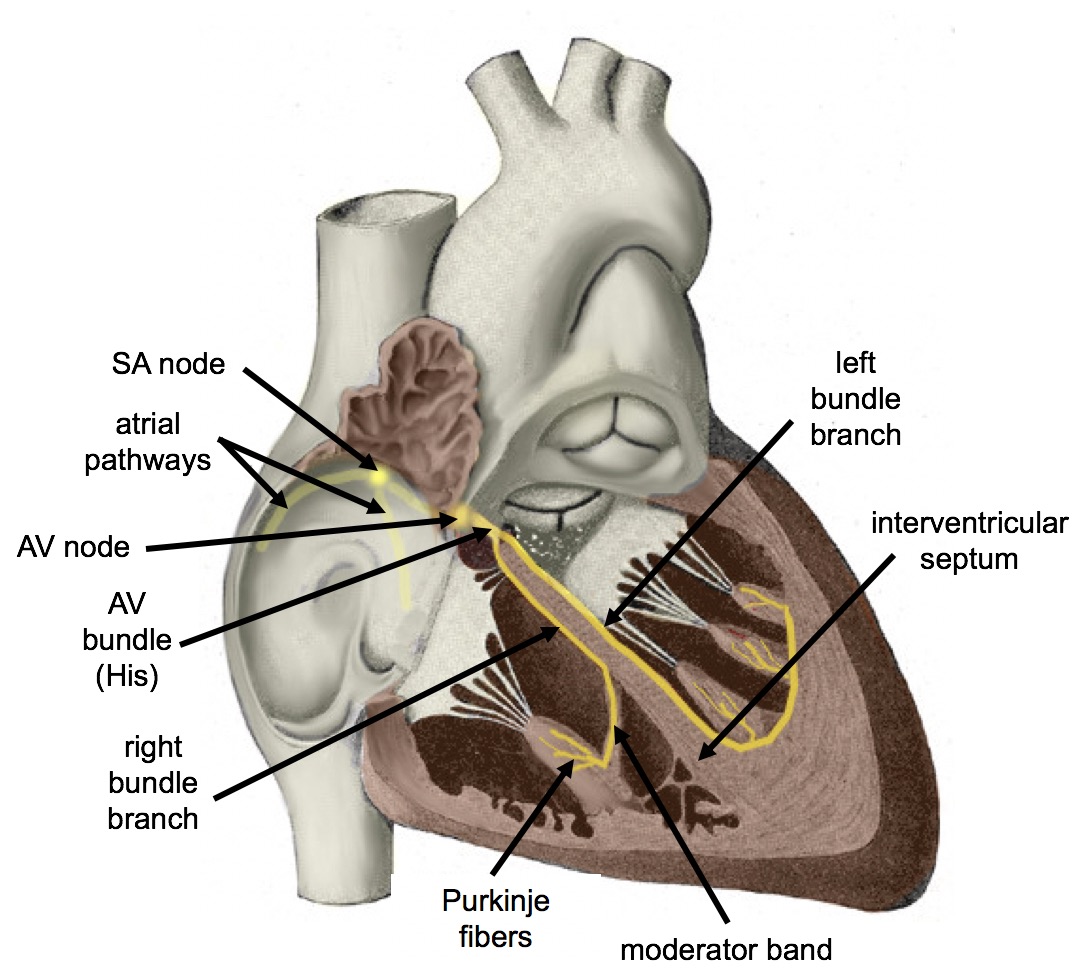
- Atrioventricular node
A part of the electrical conduction system of the heart that coordinates the top of the heart. It electrically connects the atria and ventricles.
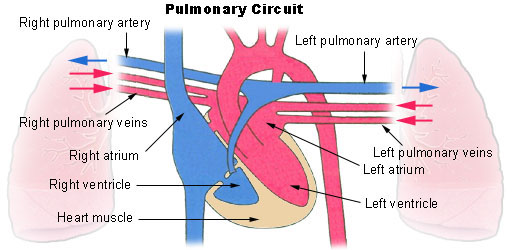
- Atrium
One of the two upper chambers of the heart that pumps blood to the ventricle below it. Plural form is atria.
- Atrophy
The decrease in the size of a structure, such as a decrease in the size of a muscle through non-use.
- Autoimmune disease
A type of disease, such as Type 1 Diabetes, in which the immune system attacks the body’s own cells as though they were pathogens.
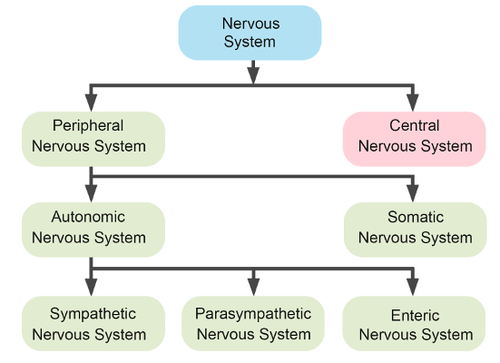
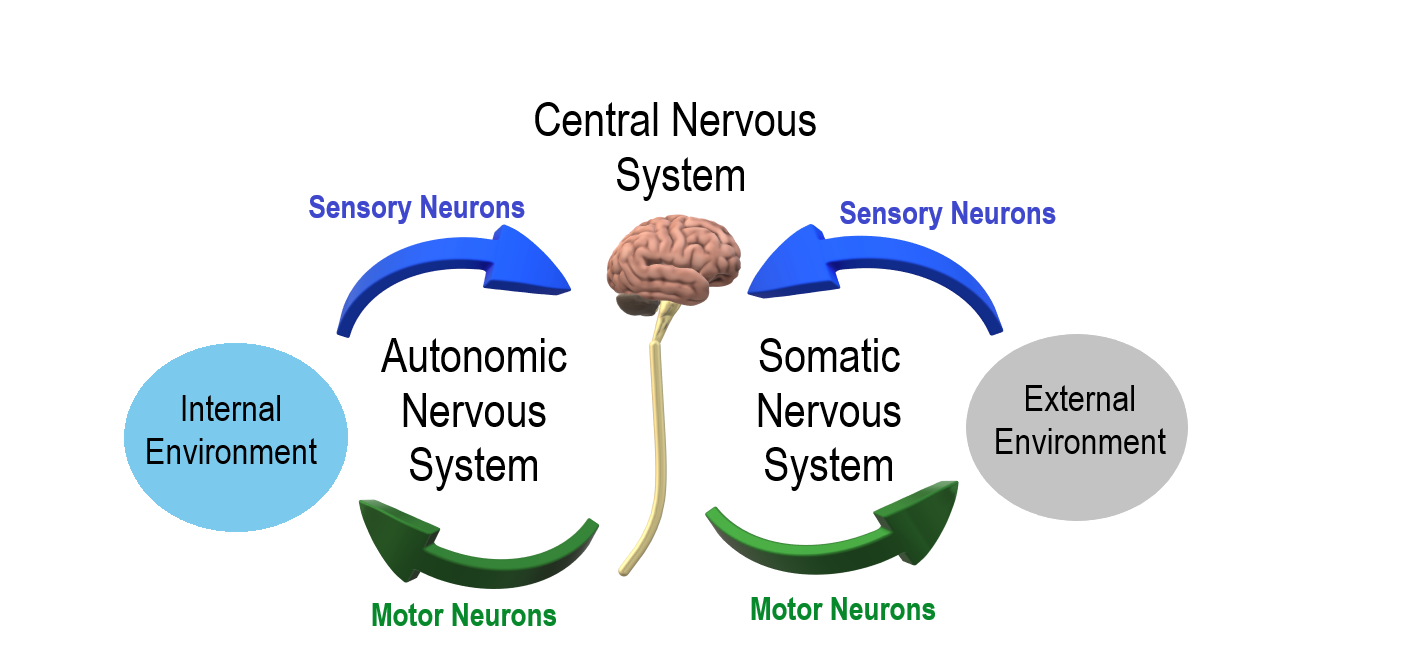
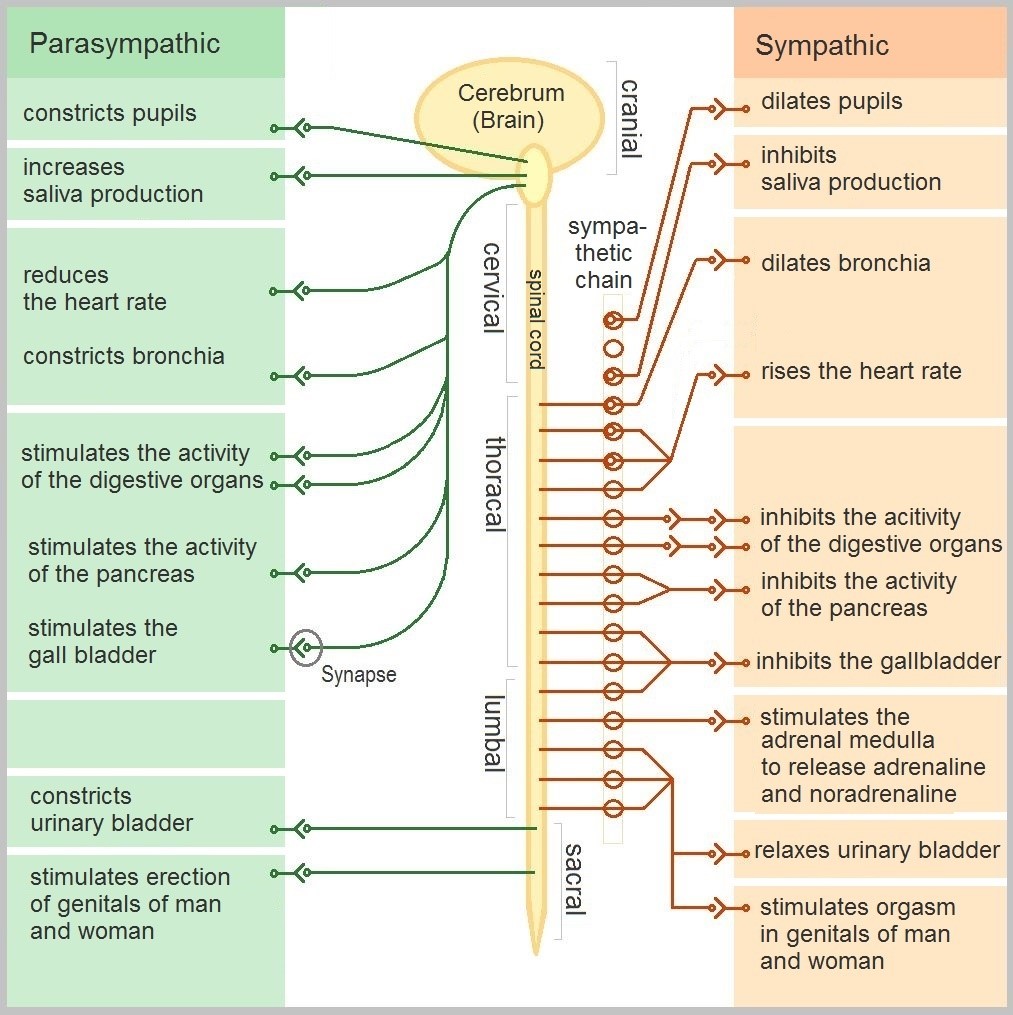
- Autonomic nervous system
A division of the peripheral nervous system that controls involuntary activities.
- Autonomic nervous system
division of the peripheral nervous system that controls involuntary activities
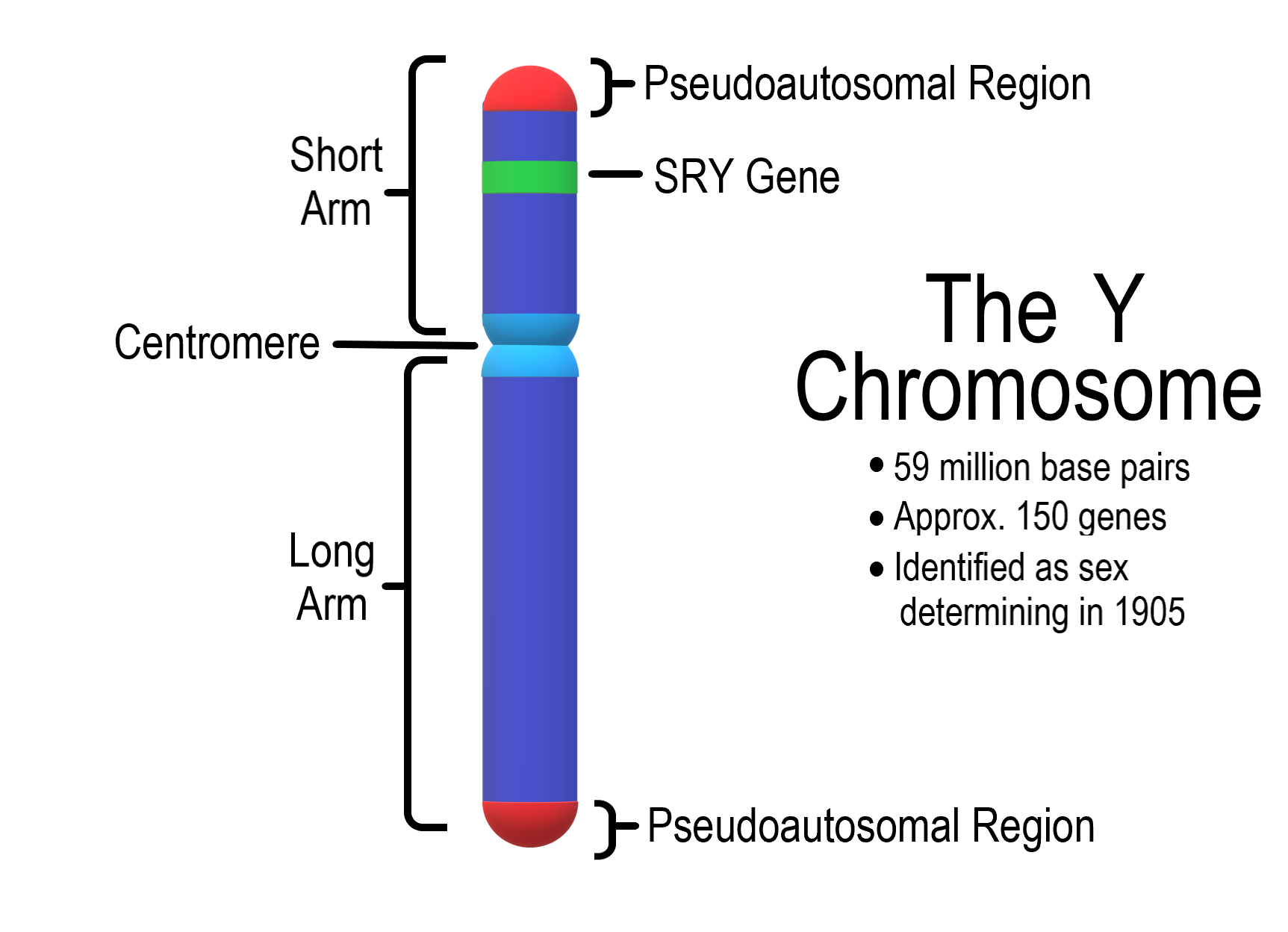
- Autosomes
Any chromosome that is not a sex chromosome.
- Autotroph
An organism that produces complex organic compounds (such as carbohydrates, fats, and proteins) from simple substances present in its surroundings, generally using energy from light (photosynthesis) or inorganic chemical reactions (chemosynthesis).
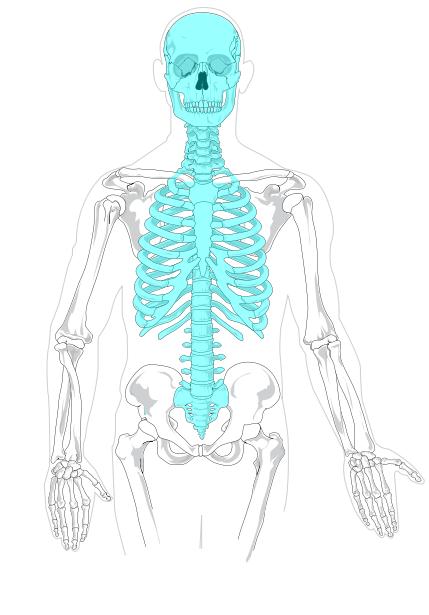
- Axial skeleton
A division of the skeleton that includes the skull, rib cage, and vertebral column.
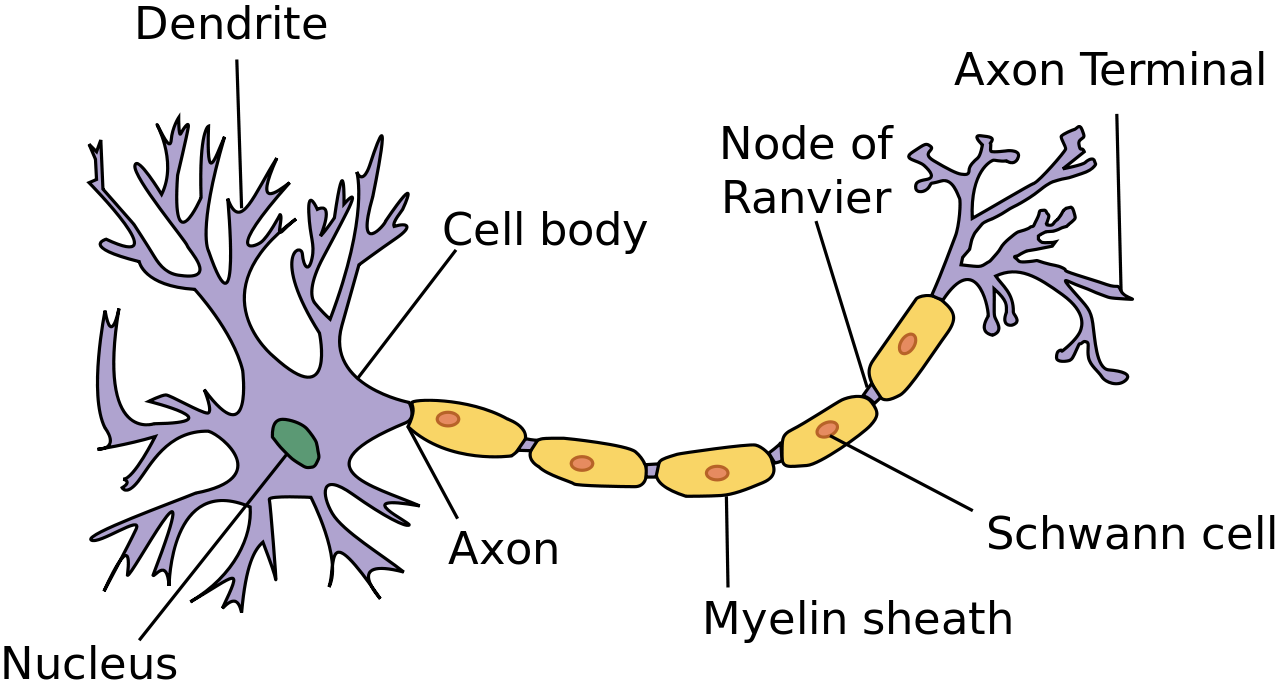
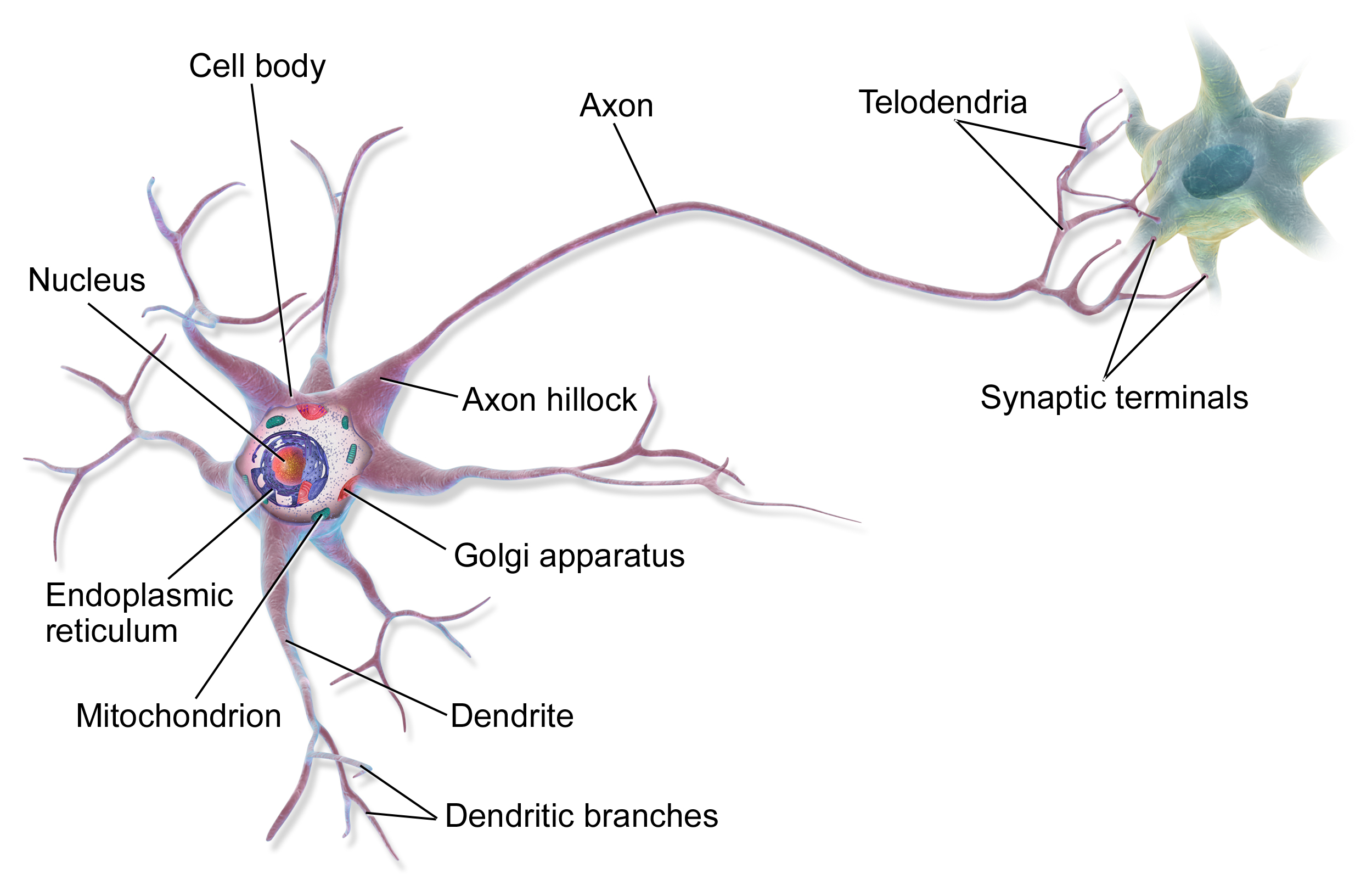
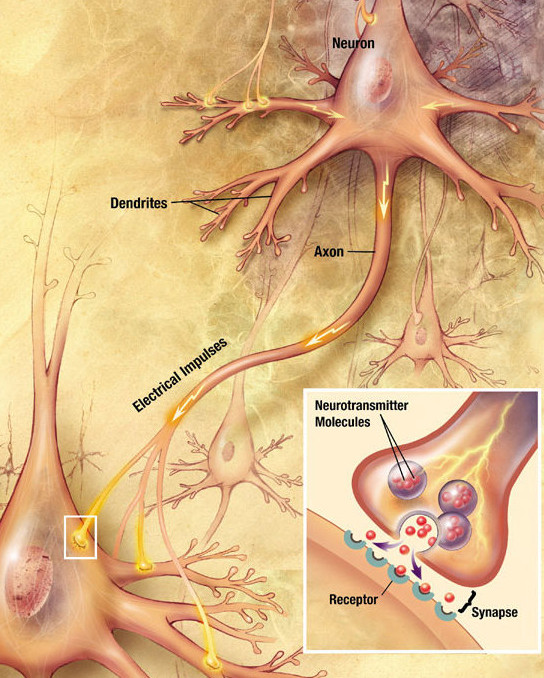
- Axon
A long extension of the cell body of a neuron that transmits nerve impulses to other cells.
- B cell
A type of white blood cell and, specifically, a type of lymphocyte.
Many B cells mature into what are called plasma cells that produce antibodies (proteins) necessary to fight off infections while other B cells mature into memory B cells.
- Bacteria
Any member of a large group of unicellular microorganisms which have cell walls but lack organelles and an organized nucleus, including some which can cause disease.
- Balance
The ability to sense and maintain an appropriate body position.
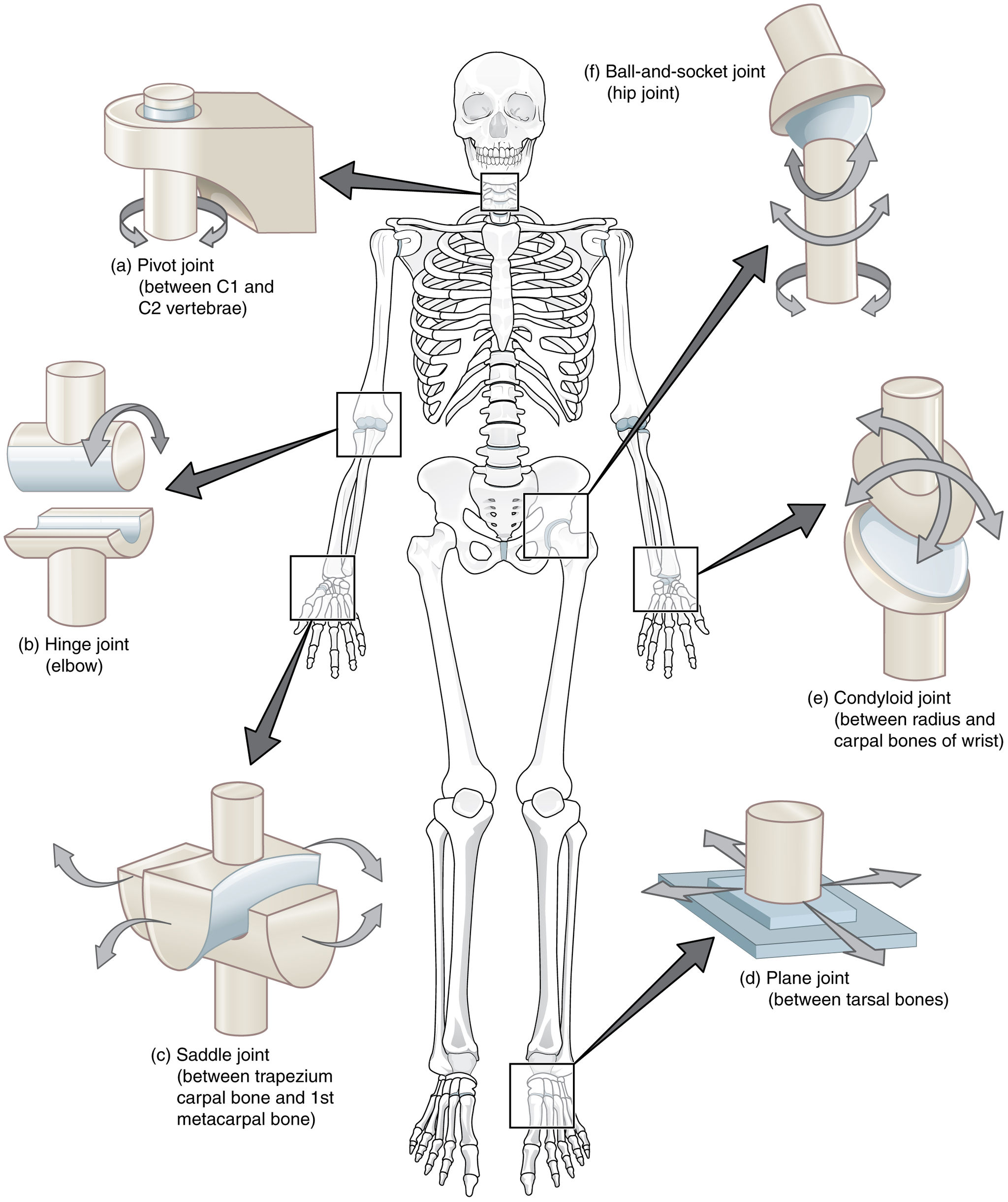
- Ball-and-socket joint
A natural or manufactured joint or coupling, such as the hip joint, in which a partially spherical end lies in a socket, allowing multi-directional movement and rotation.
- Barr body
The inactive X chromosome in a female somatic cell, rendered inactive in a process called lyonization.
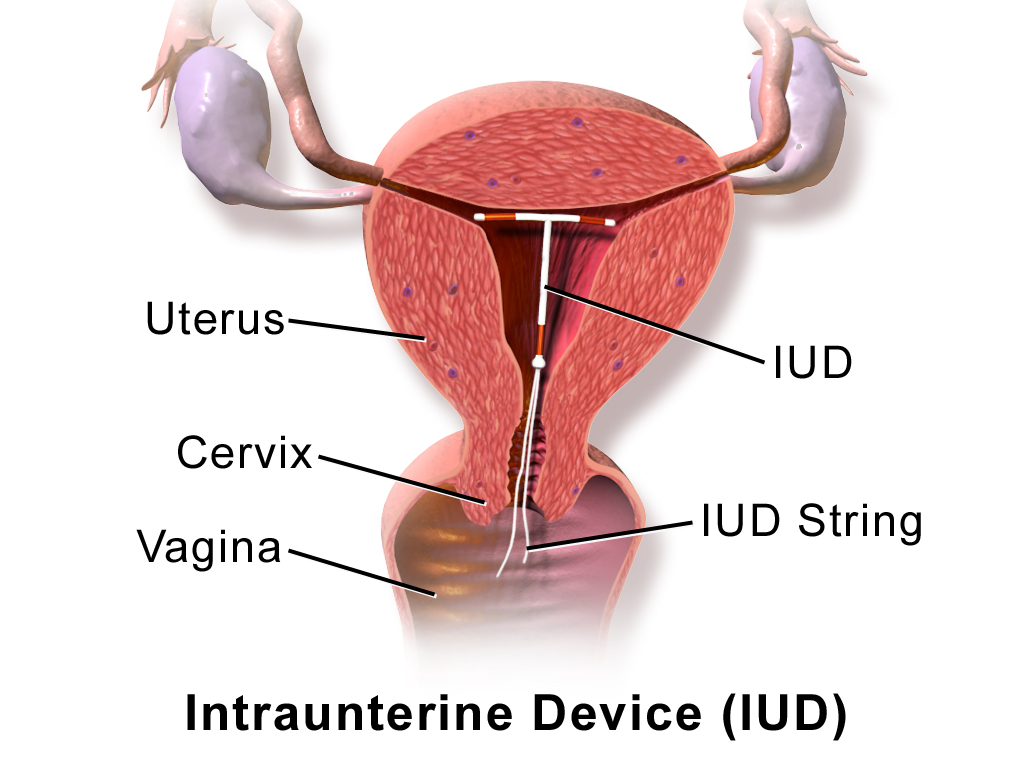
- Barrier method
A type of contraception in which a device such as a condom or diaphragm is used to physically block sperm from entering the uterus.
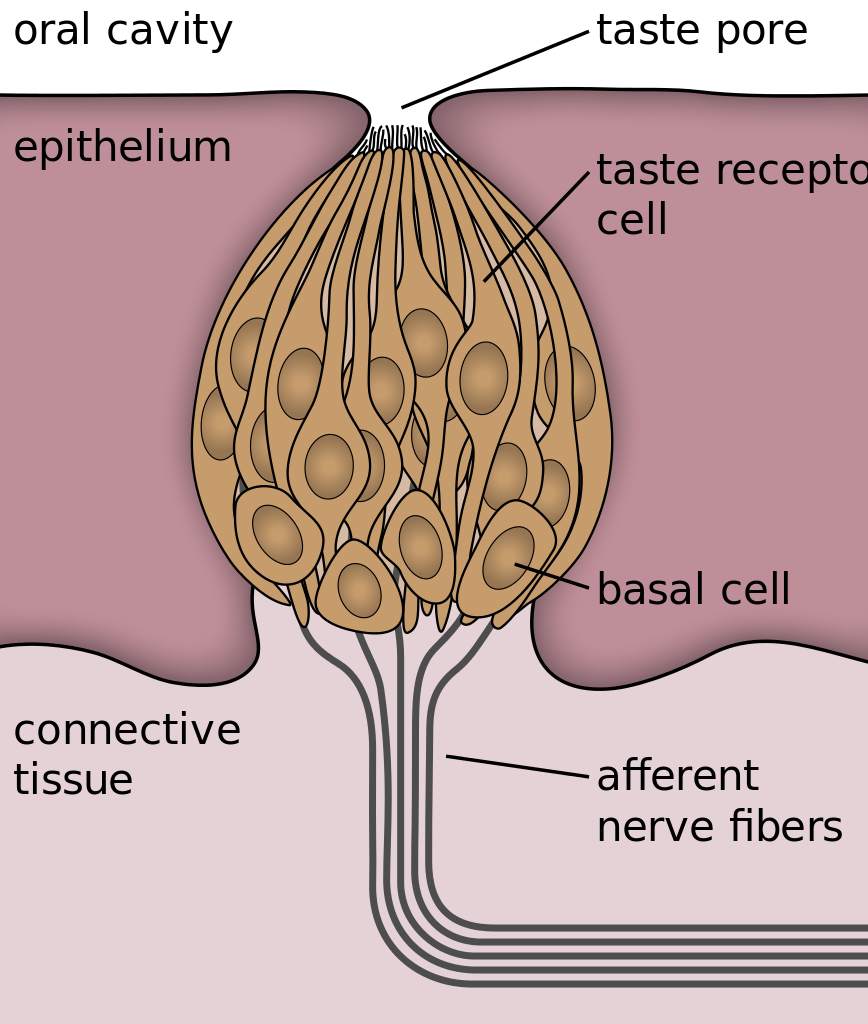
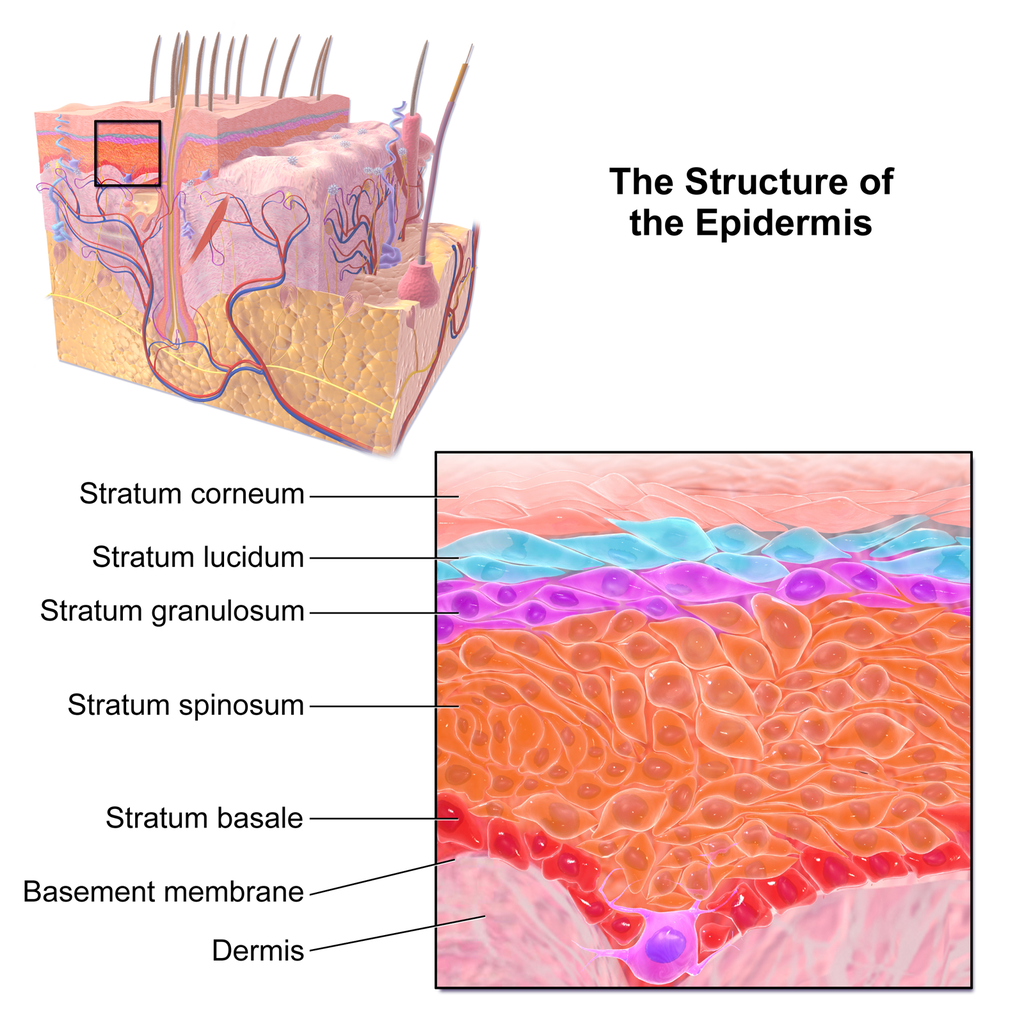
- Basal cell
Found at the bottom of the epidermis — the outermost layer of skin. Basal cells produce new skin cells. As new skin cells are produced, they push older cells toward the skin's surface, where the old cells die and are sloughed off.
- Basal cell carcinoma
The most common type of skin cancer that occurs in basal cells of the epidermis and rarely metastasizes.
- Basal metabolic rate
The number of calories required to keep your body functioning at rest.
- Base
Substances that, in aqueous solution, release hydroxide ions, are slippery to the touch, can taste bitter if an alkali.
- Basement membrane
A thin, fibrous, extracellular matrix that separates the lining of an internal or external body surface from underlying connective tissue.
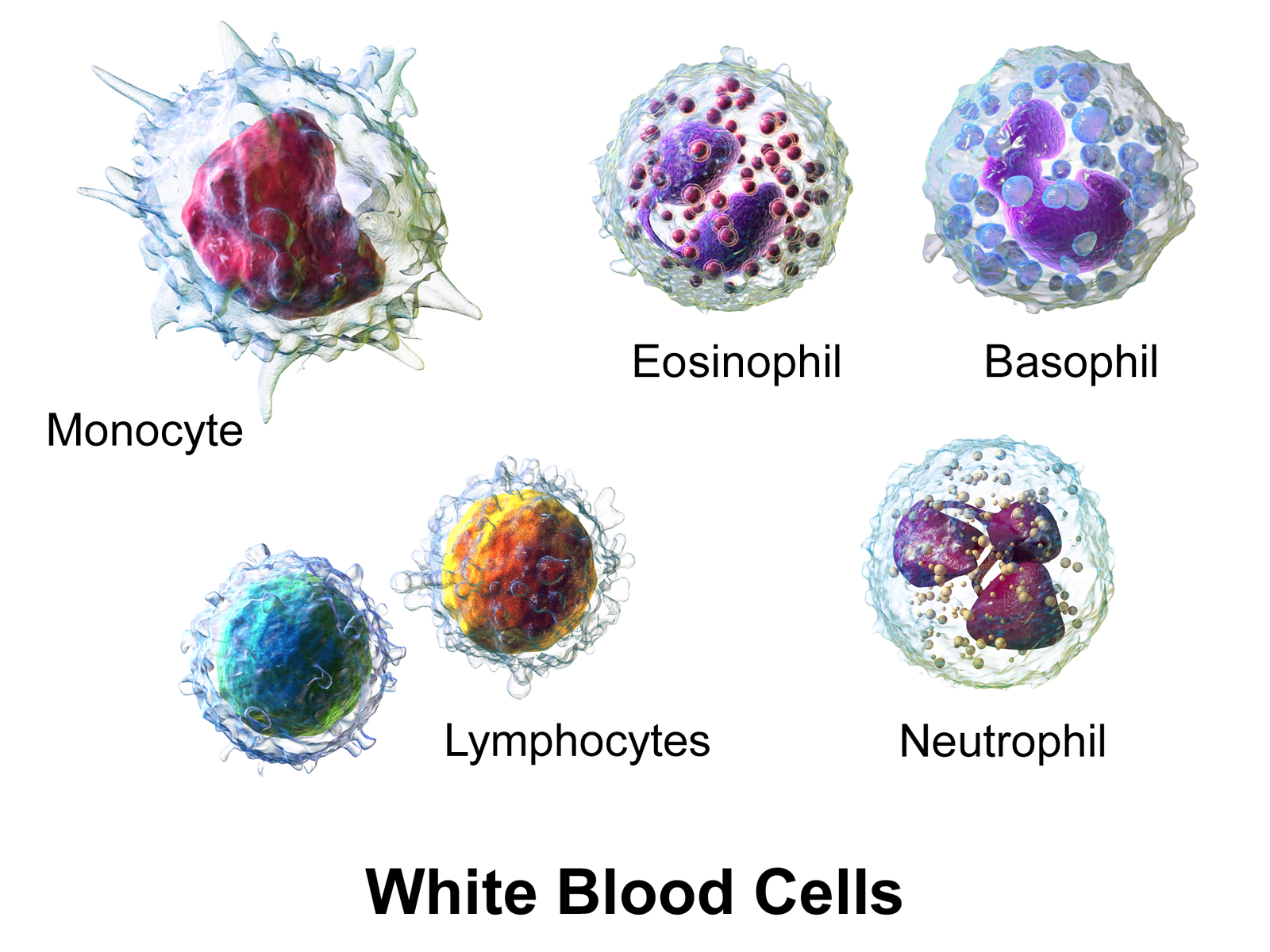
- Basophil
A type of immune cell that has granules (small particles) with enzymes that are released during allergic reactions and asthma. A basophil is a type of white blood cell and a type of granulocyte.
- Bergmann's rule
An ecogeographical rule that states that within a broadly distributed taxonomic clade, populations and species of larger size are found in colder environments, and species of smaller size are found in warmer regions.
- Bicuspid atrioventricular valve
A valve which permits blood to flow one way only, from the left atrium into the left ventricle This valve is more commonly called the mitral valve because it has two flaps (cusps) and looks like a bishop's miter or headdress.
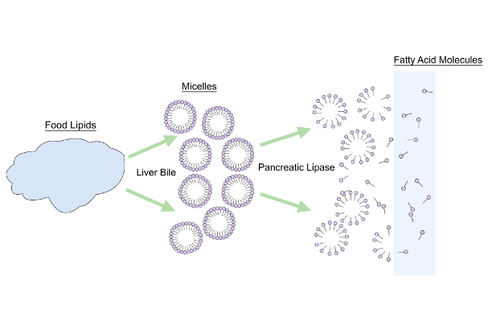
- Bile
Fluid produced by the liver and stored in the gall bladder that is secreted into the small intestine to help digest lipids and neutralize acid from the stomach.
- Bilirubin
A brown pigment secreted into bile by the liver that is a byproduct of catabolism of dead red blood cells and is excreted in feces by the large intestine.
- Biochemical reaction
The transformation of one molecule to a different molecule inside a cell.
- Biodiversity
The variety of life in the world, ecosystem, or in a particular habitat.
- Biofuel
A fuel that is produced through contemporary processes from biomass, rather than a fuel produced by the very slow geological processes involved in the formation of fossil fuels, such as oil.
- Biopsy
The surgical removal of a tissue specimen for analysis in a medical laboratory, usually to diagnose cancer
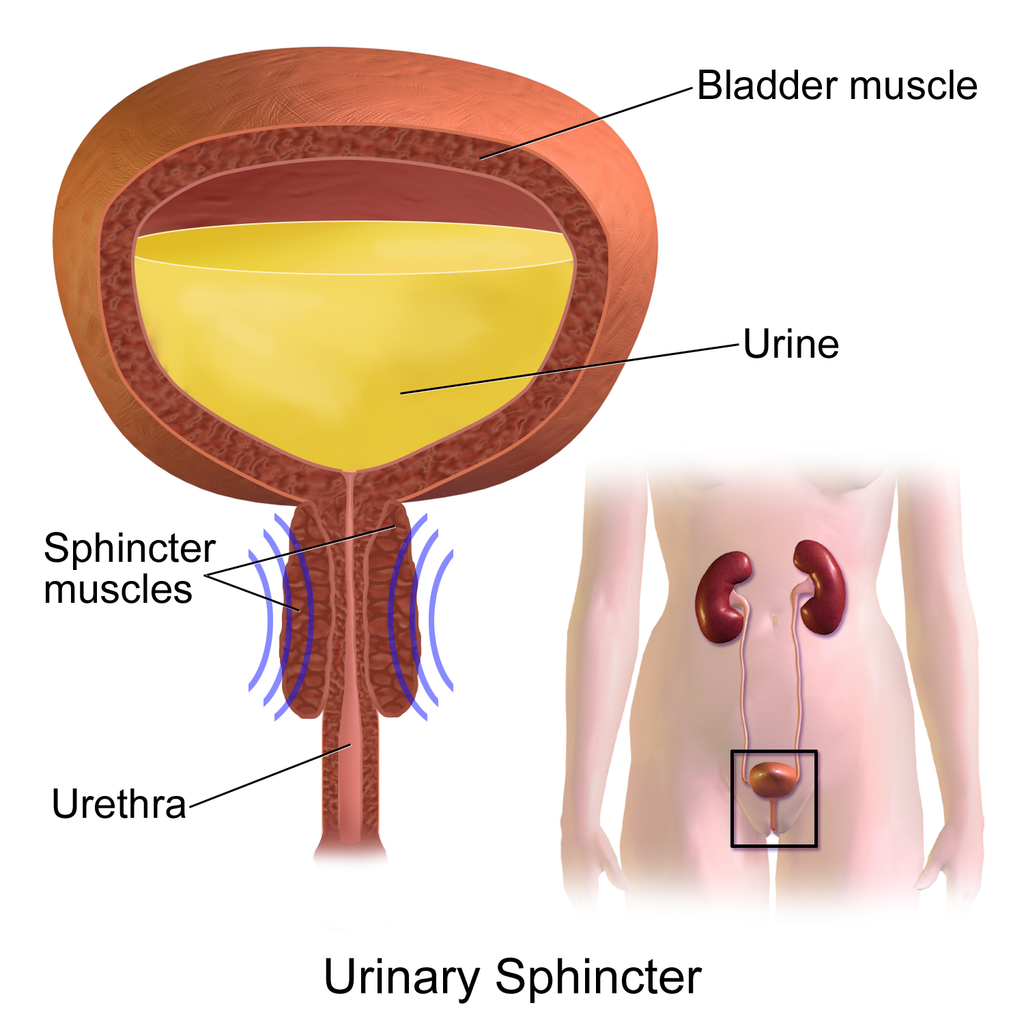
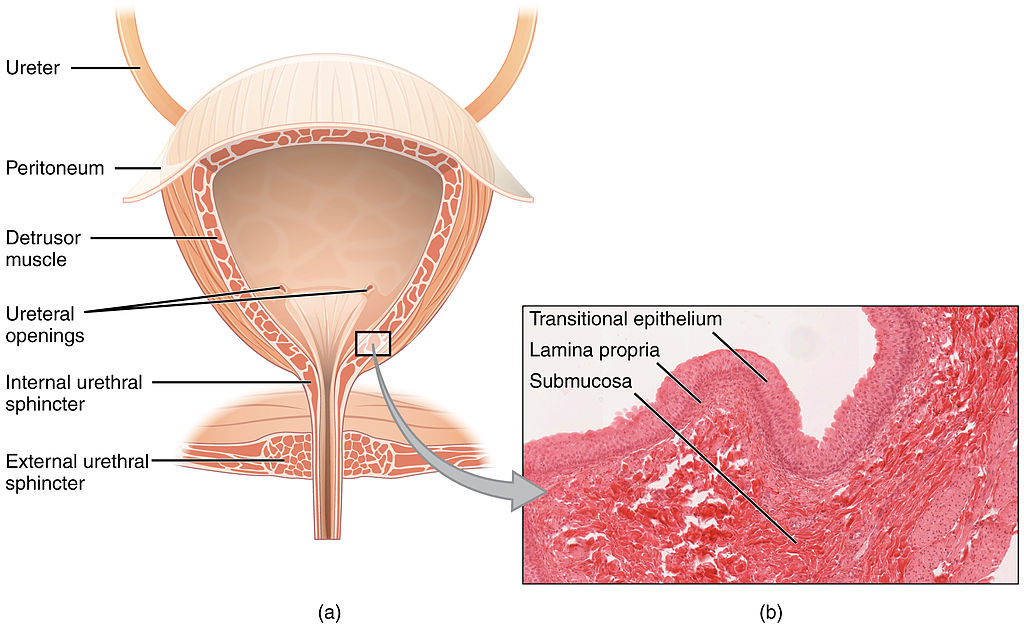
- Bladder infection
A common type of urinary tract infection in which the bladder becomes infected, usually by bacteria but occasionally by fungi.
- Blastocyst
A fluid-filled ball of cells that develops a few days after fertilization in the process of blastulation.
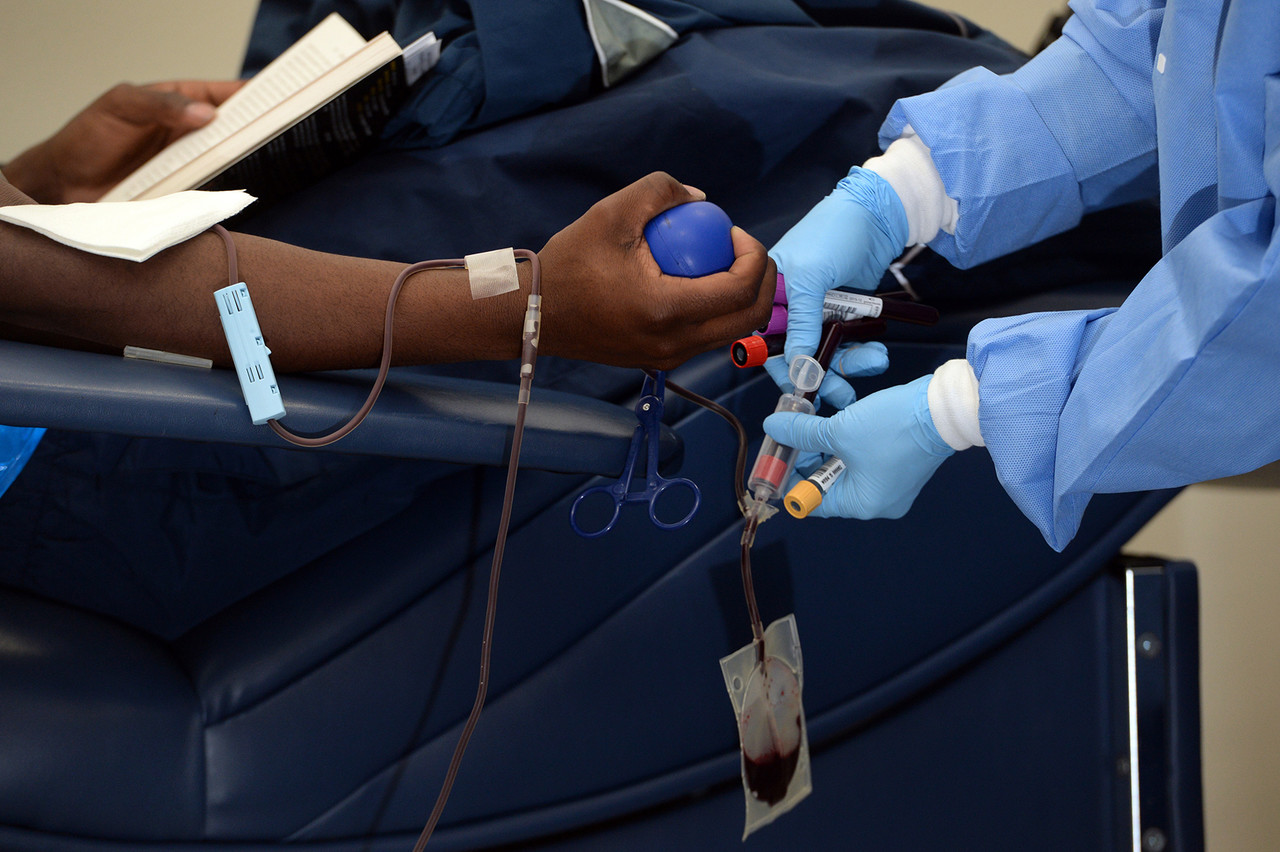
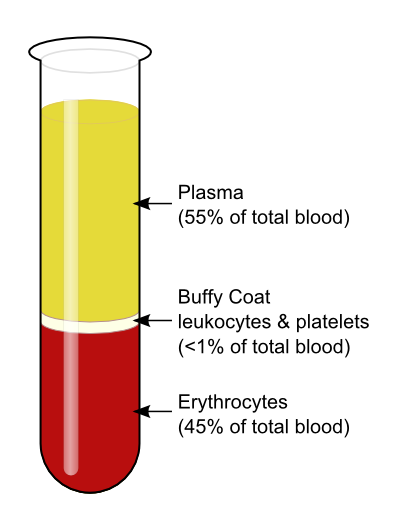
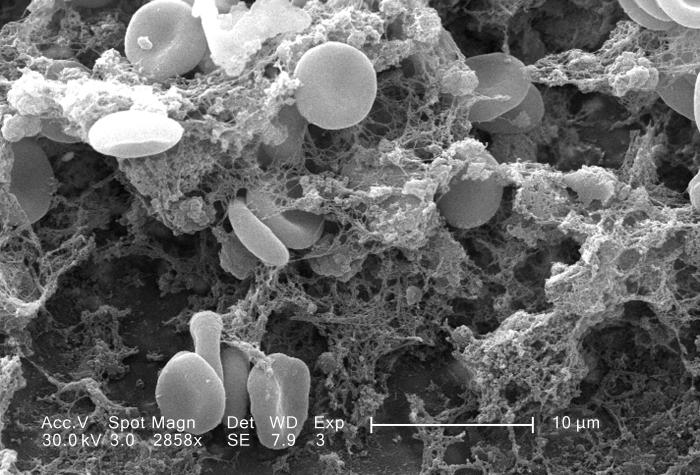
- Blood
A body fluid in humans and other animals that delivers necessary substances such as nutrients and oxygen to the cells and transports metabolic waste products away from those same cells. In vertebrates, it is composed of blood cells suspended in blood plasma.
- Blood pressure
The measure of the force exerted by circulating blood on the walls of arteries.
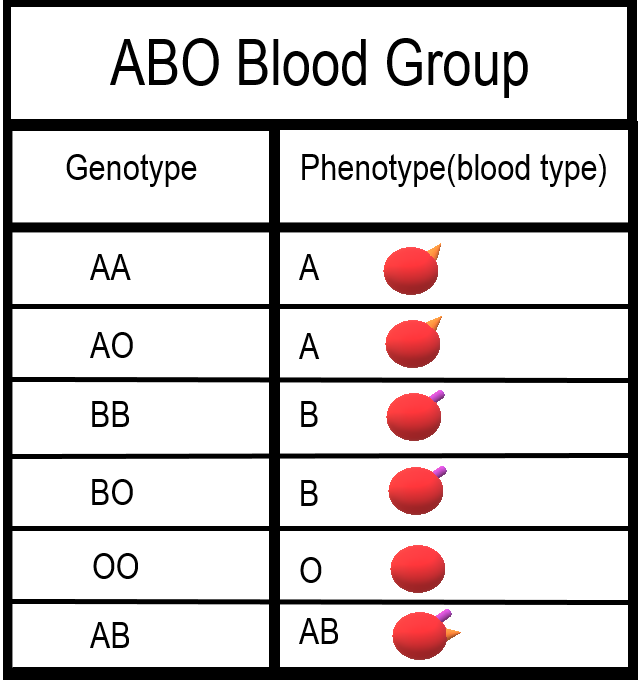
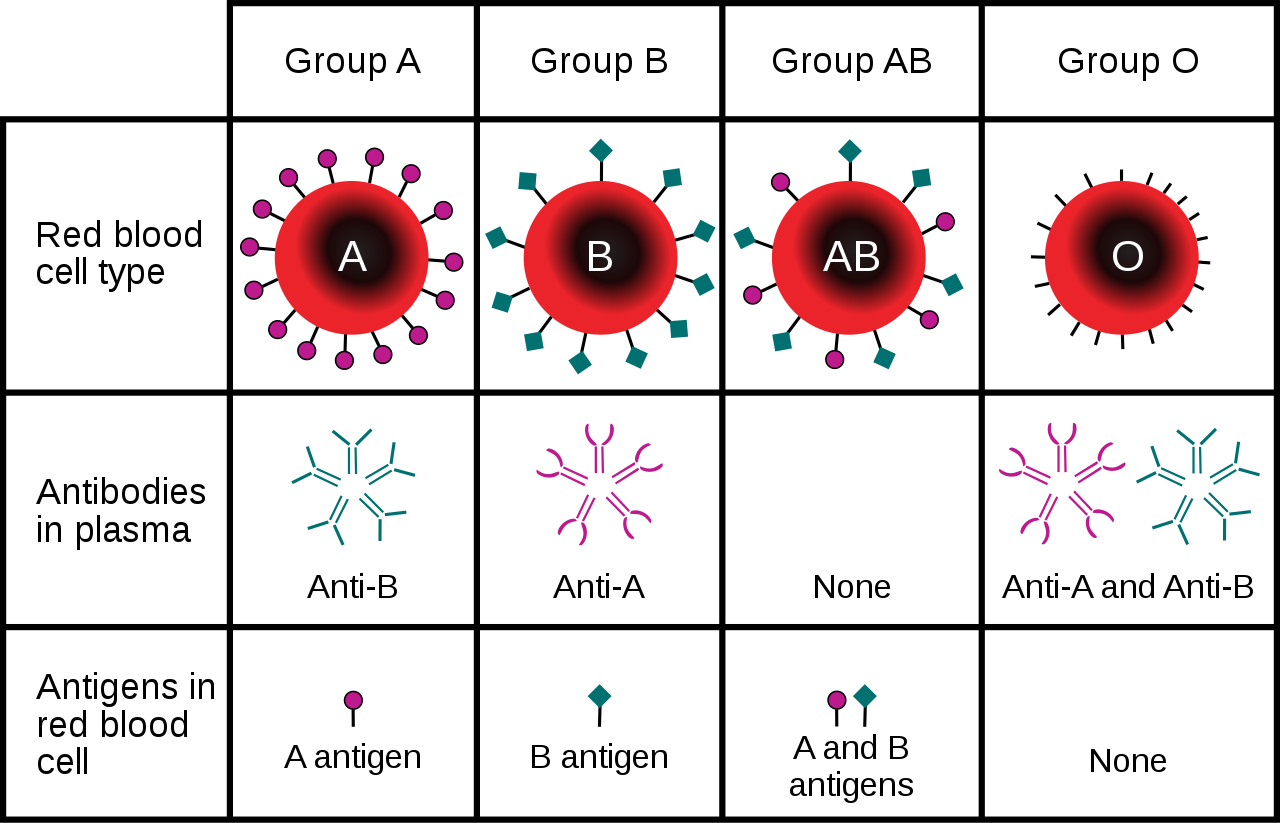
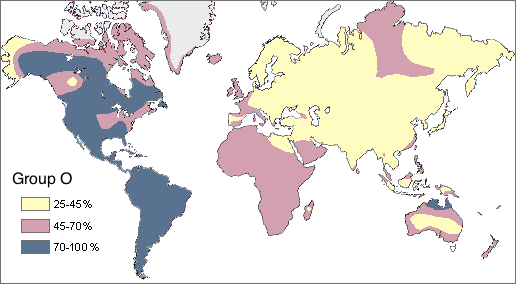
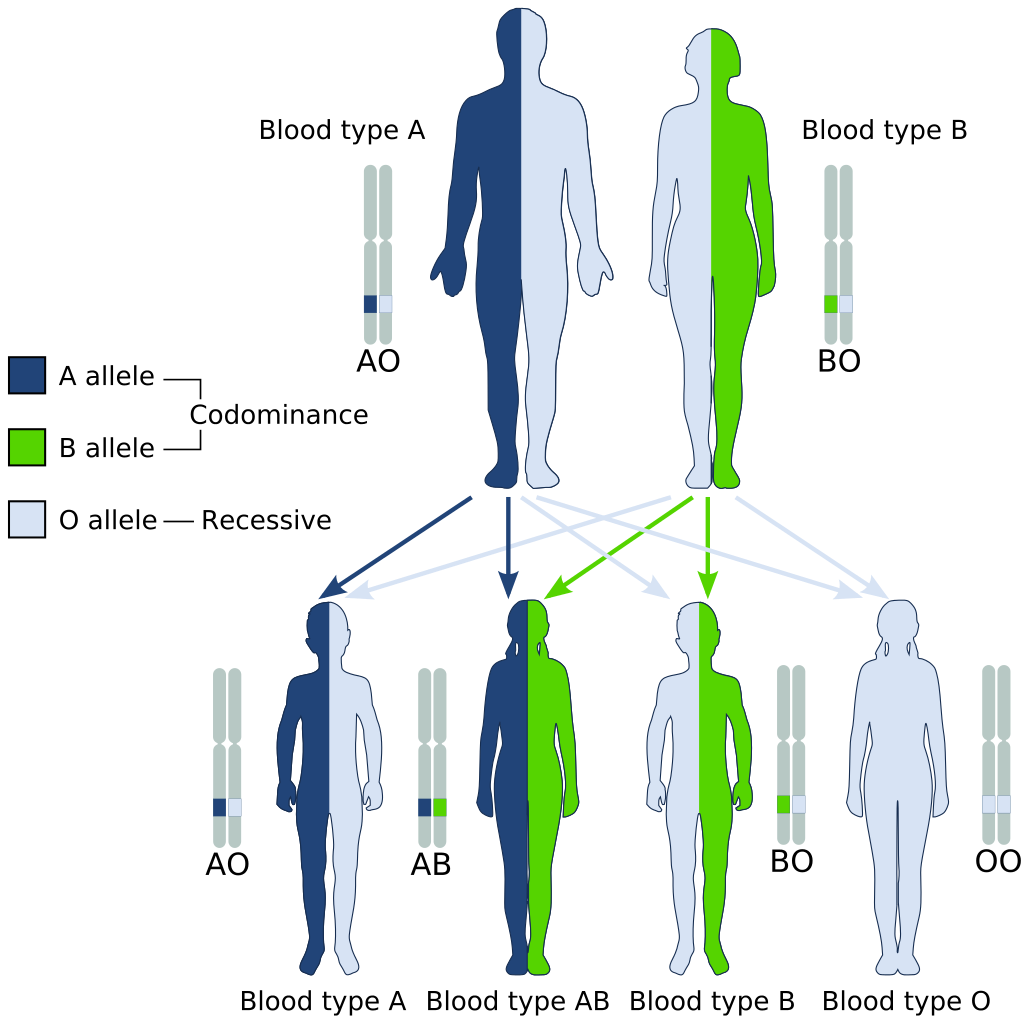
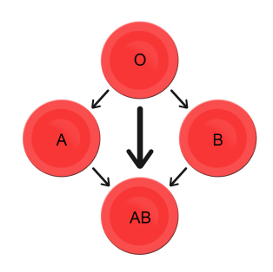
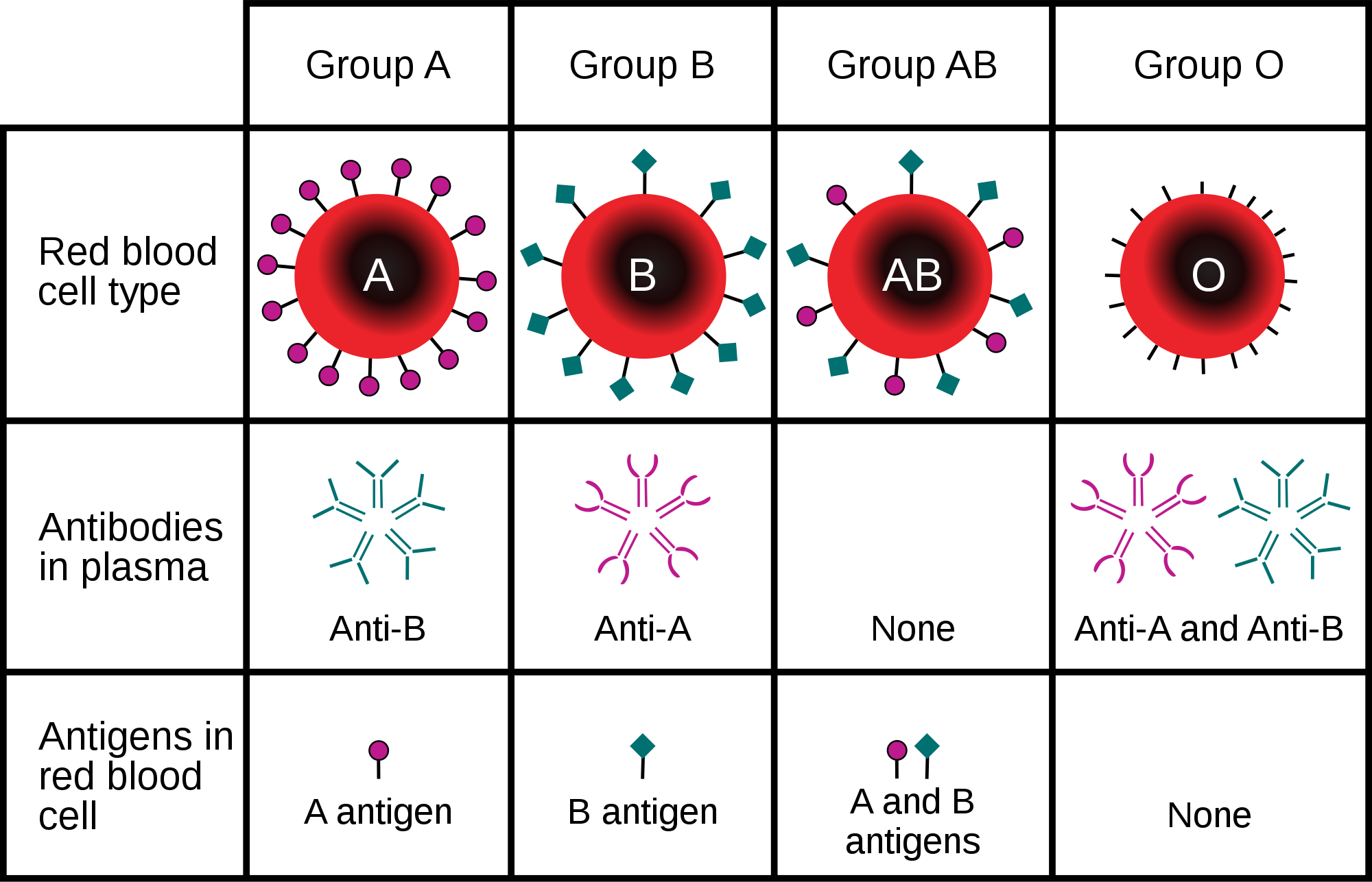
- Blood type
A classification of blood, based on the presence and absence of antibodies and inherited antigenic substances on the surface of red blood cells.
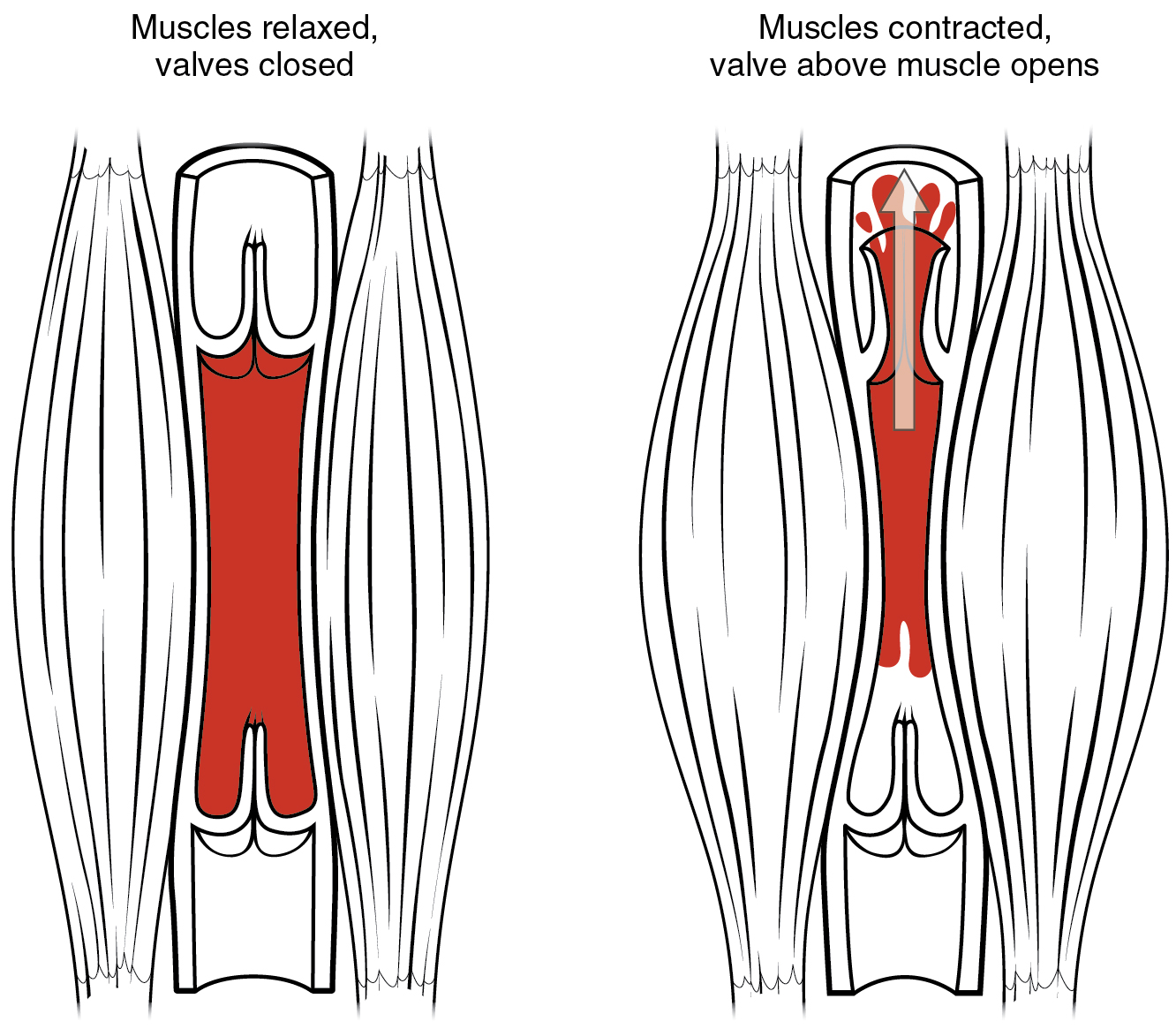
- Blood vessel
A hollow, tube-like structure through which blood flows in the cardiovascular system; vein, artery, or capillary.
- Blood-brain barrier
A highly selective membrane formed of epithelial cells that separates circulating blood from extracellular fluid in the brain and spinal cord.
- Body cavity
A fluid-filled space inside the body that holds and protects internal organs.
- Bolus
A lump of swallowed food.
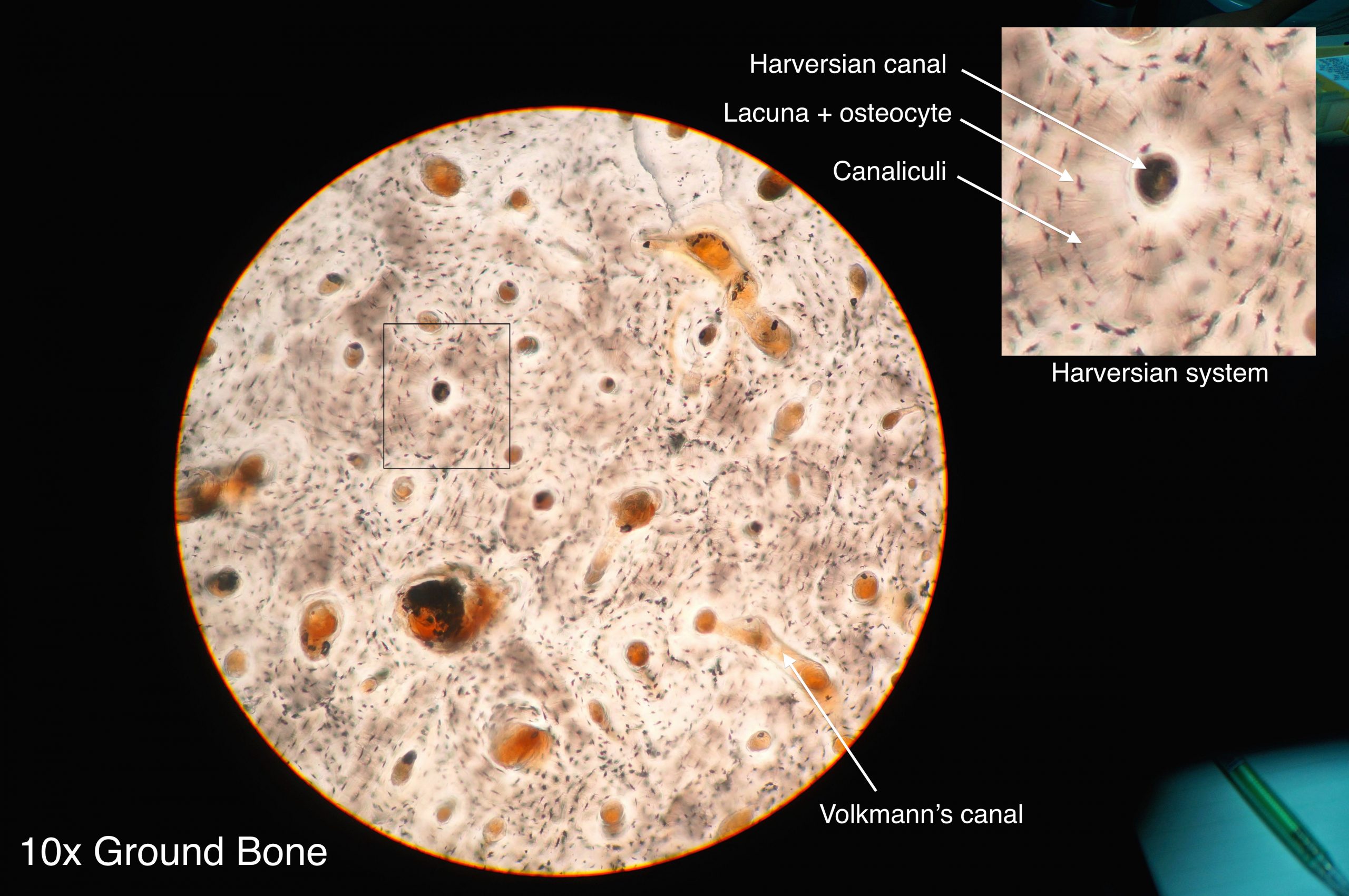
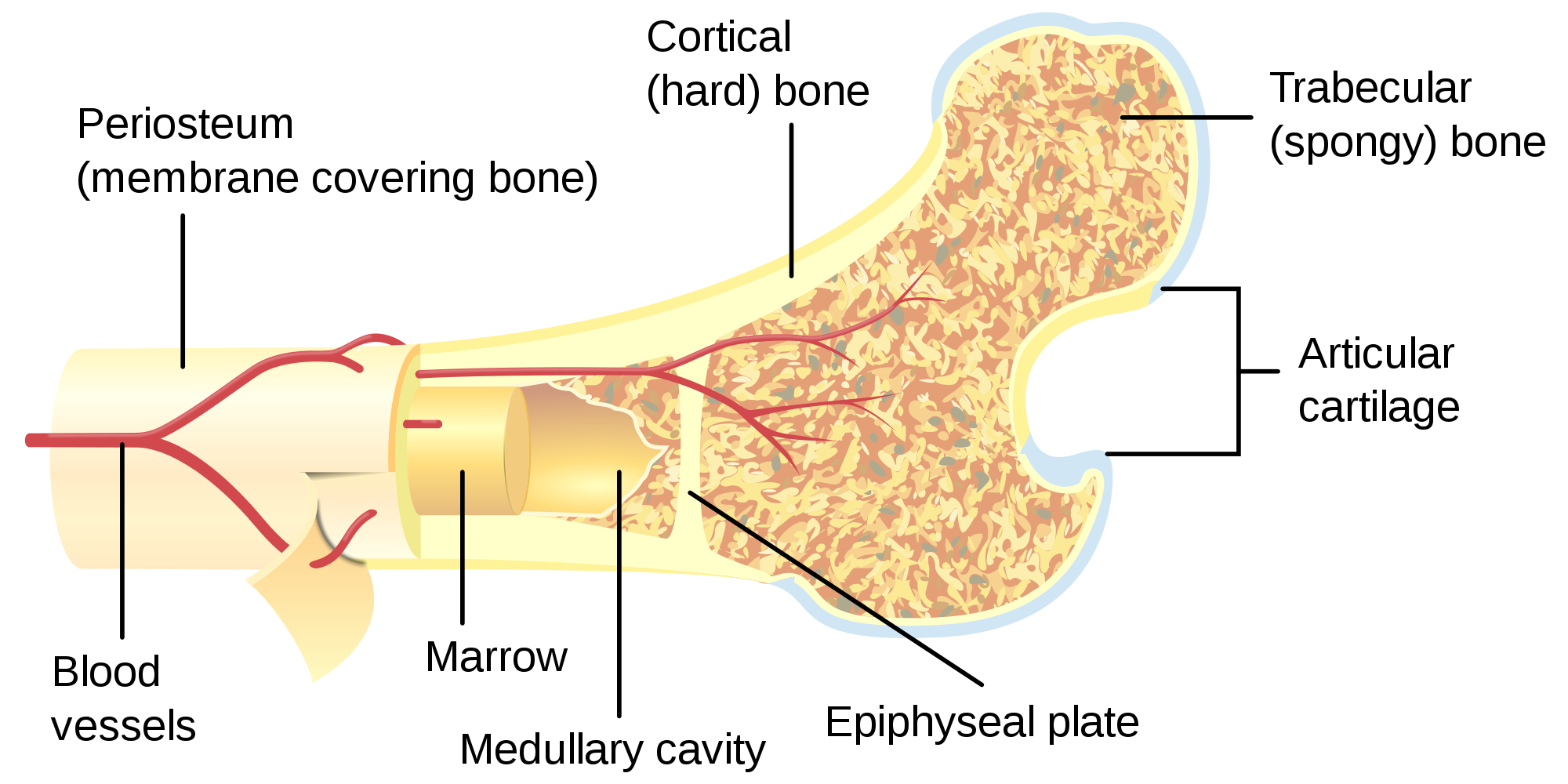
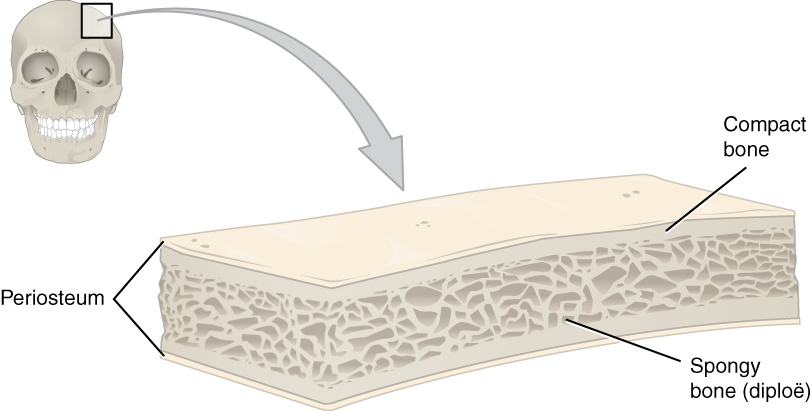
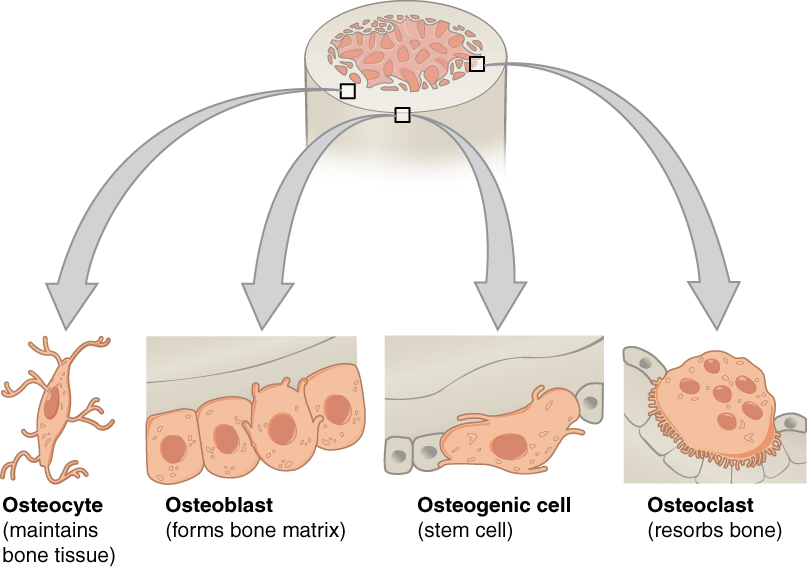
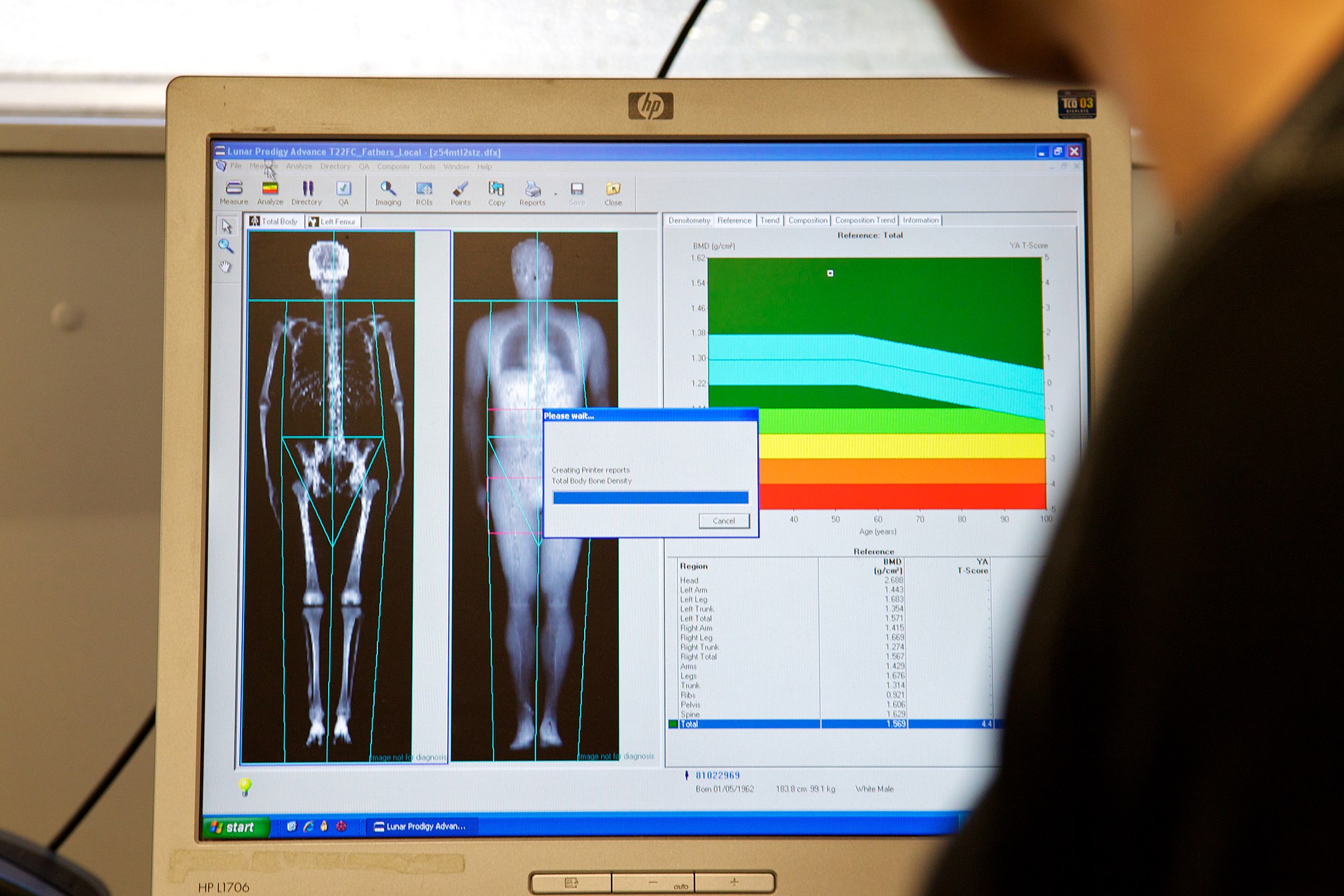
- Bone
A rigid organ that constitutes part of the vertebrate skeleton in animals.
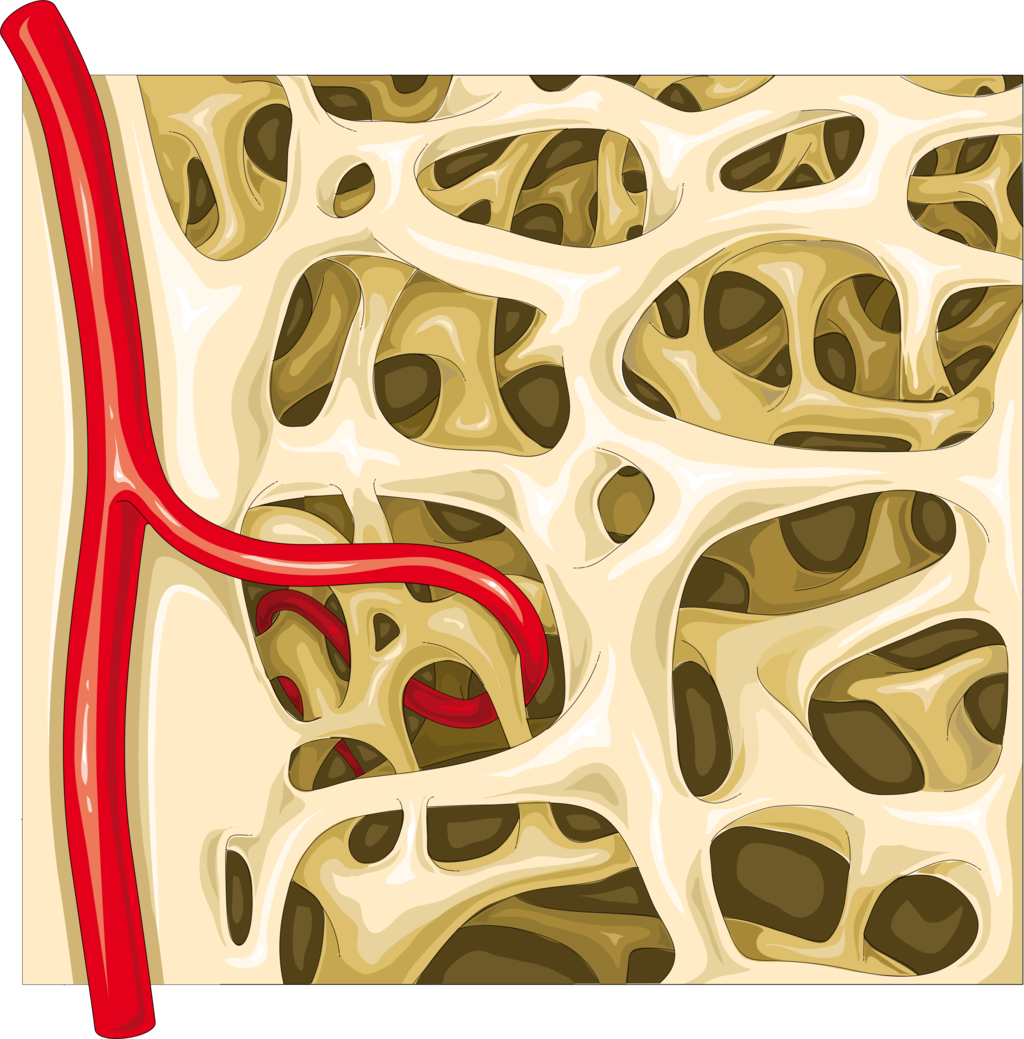
- Bone marrow
A soft connective tissue in spongy bone that produces blood cells.
- Bone remodeling
The continuous, lifelong process in which existing bone is resorbed by osteoclasts and new bone is made by osteoblasts.
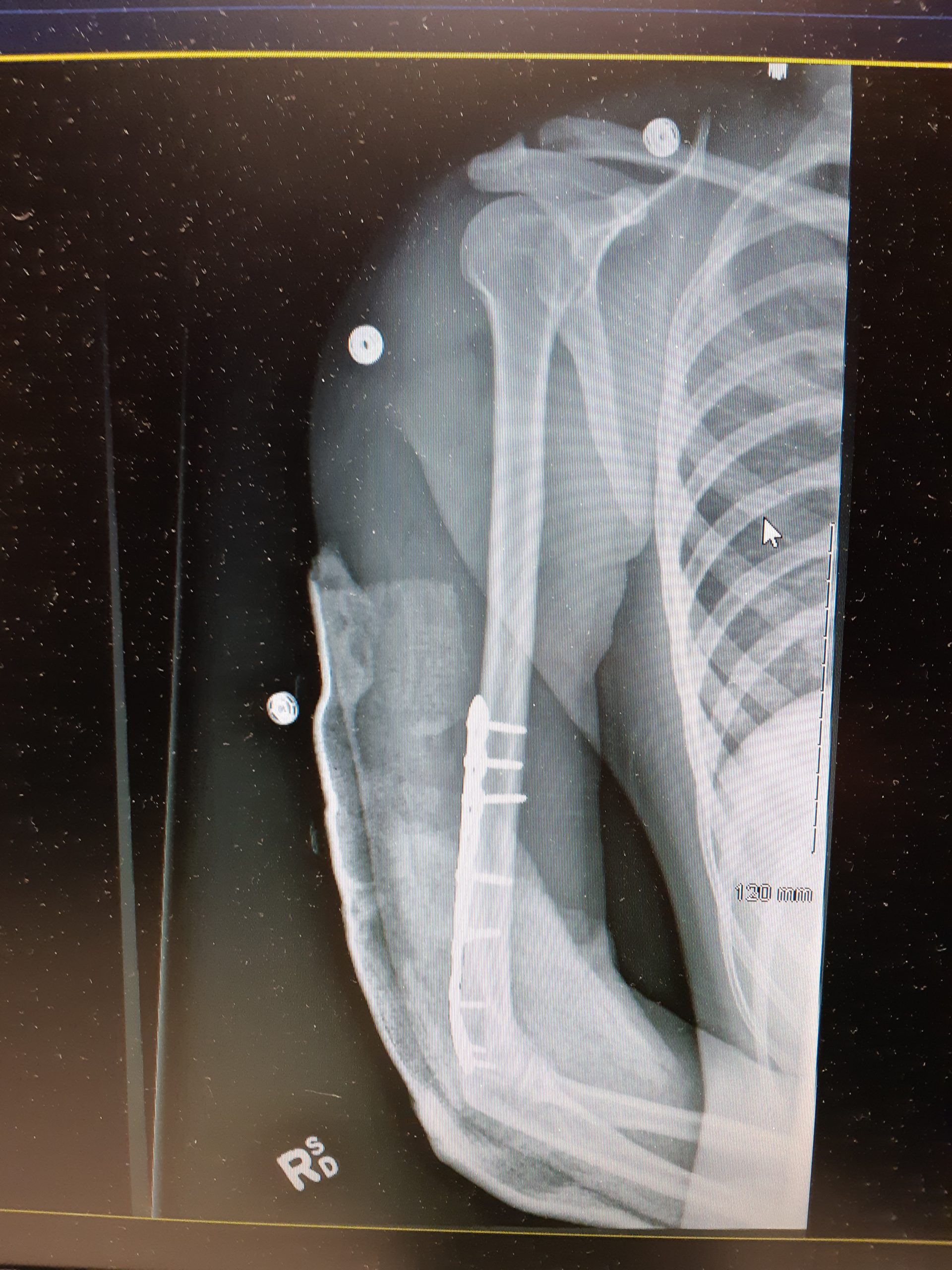
- Bone repair
The process in which bone heals itself following a bone fracture.
- Bone tissue
The hard connective tissue in bones that consists mainly of mineralized collagen matrix; also called osseous tissue.
- Botox
A drug prepared from the bacterial toxin botulin, used medically to treat certain muscular conditions and cosmetically to remove wrinkles by temporarily paralyzing facial muscles.
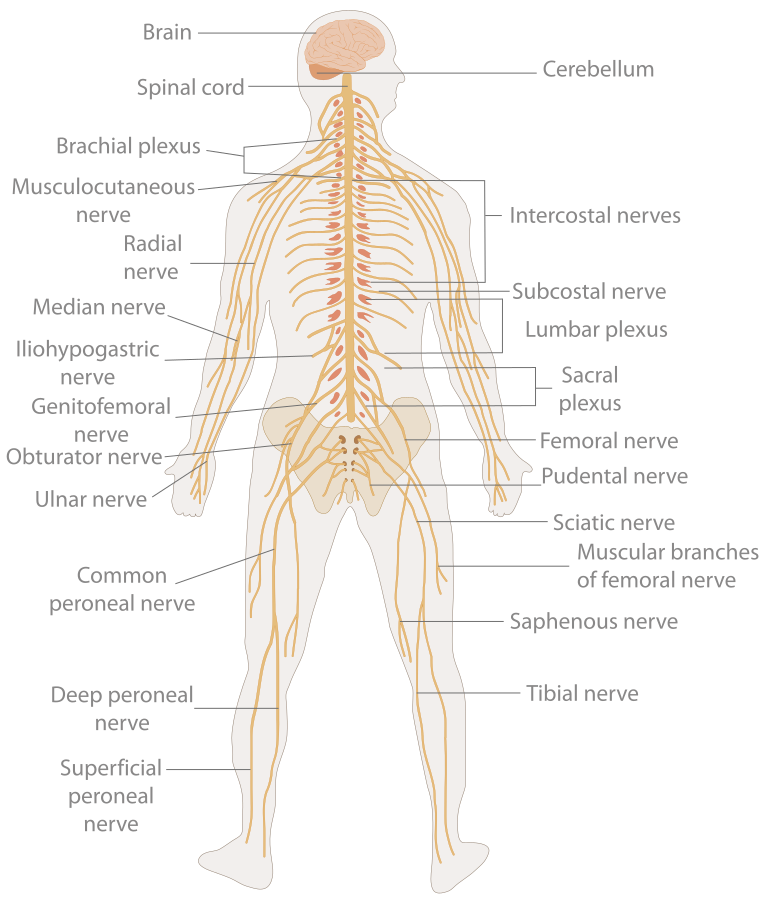
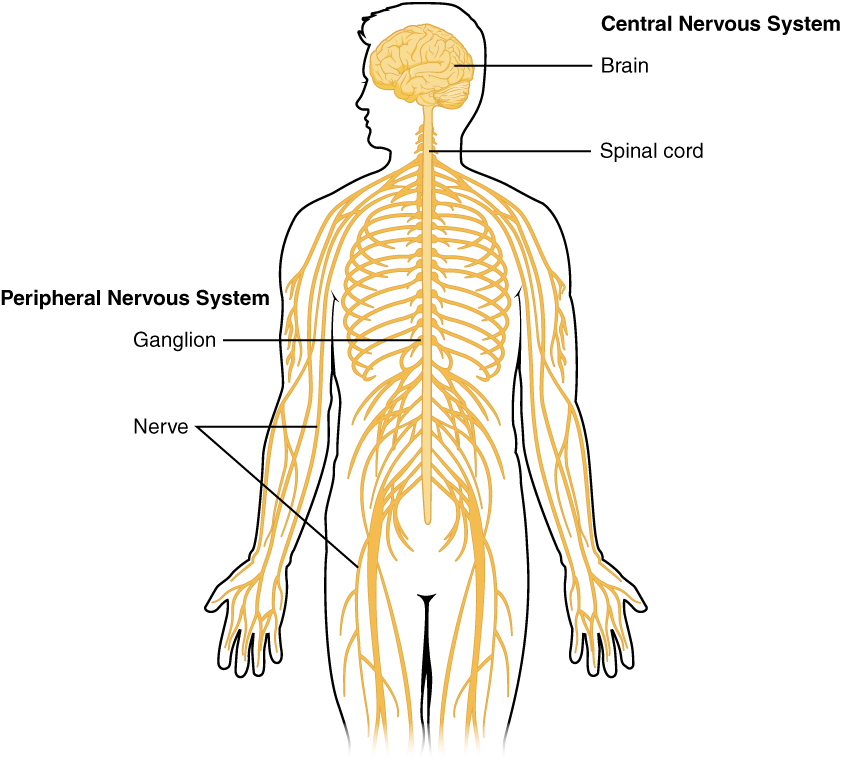
- Brain
The central nervous system organ inside the skull that is the control center of the nervous system.
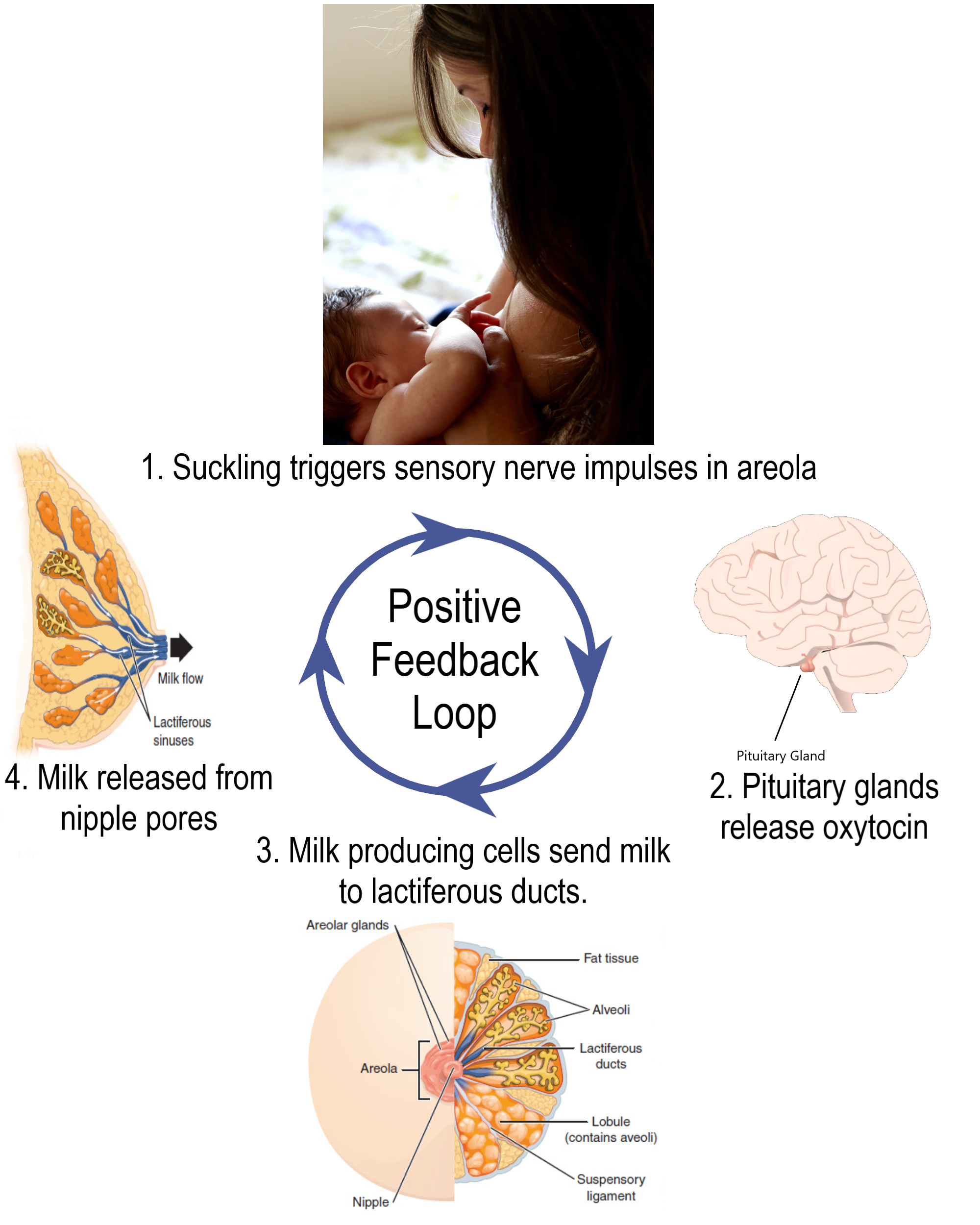
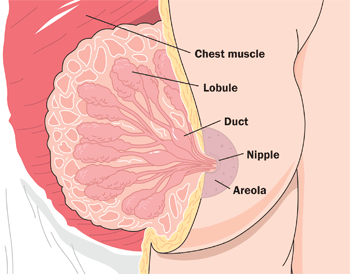
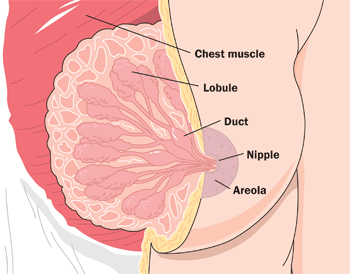
- Breast
Refers to the front of the chest or, more specifically, to the mammary gland. The mammary gland is a milk producing gland. It is composed largely of fat.
- Breeding population
A population within which free interbreeding takes place and evolutionary change may appear and be preserved.
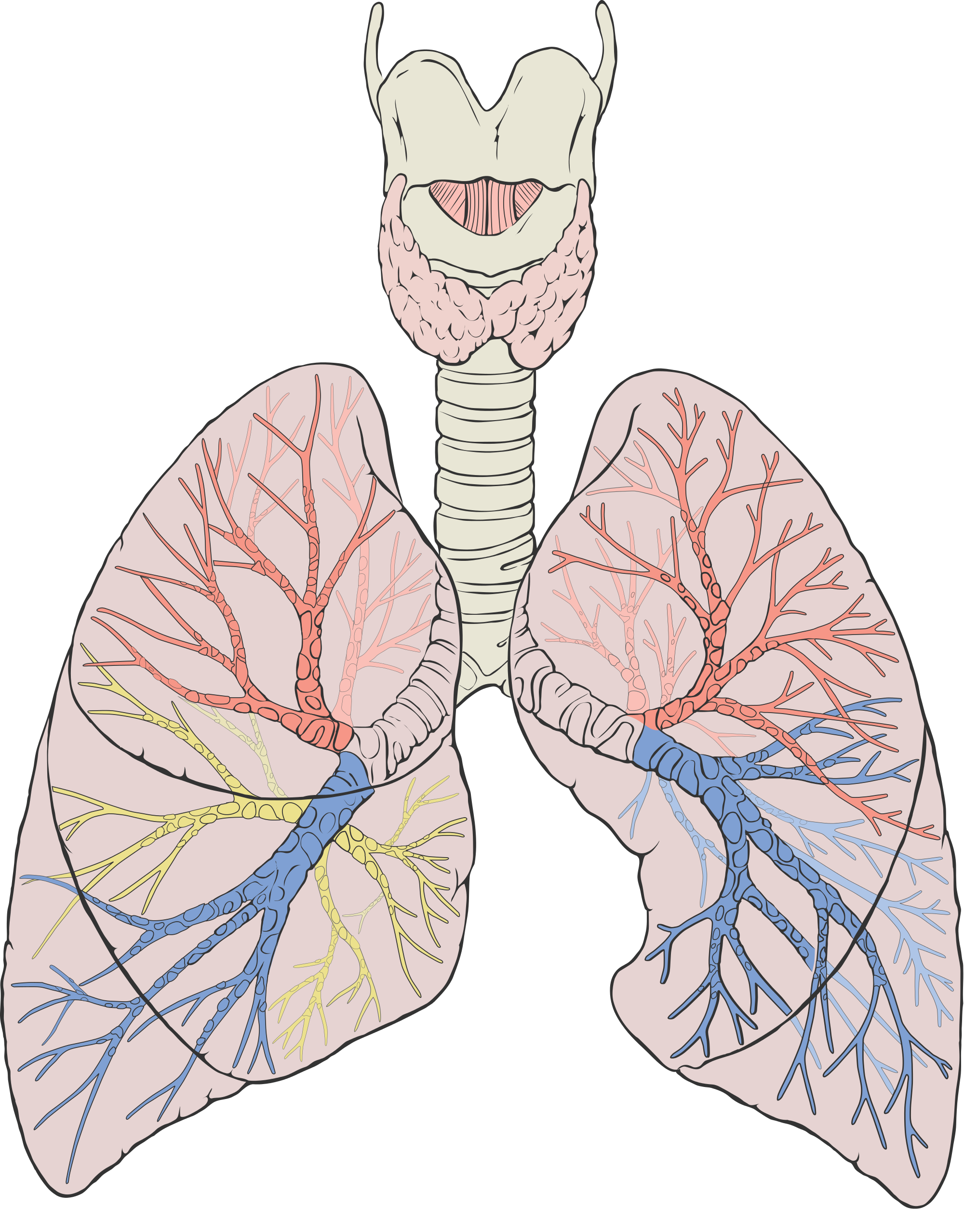
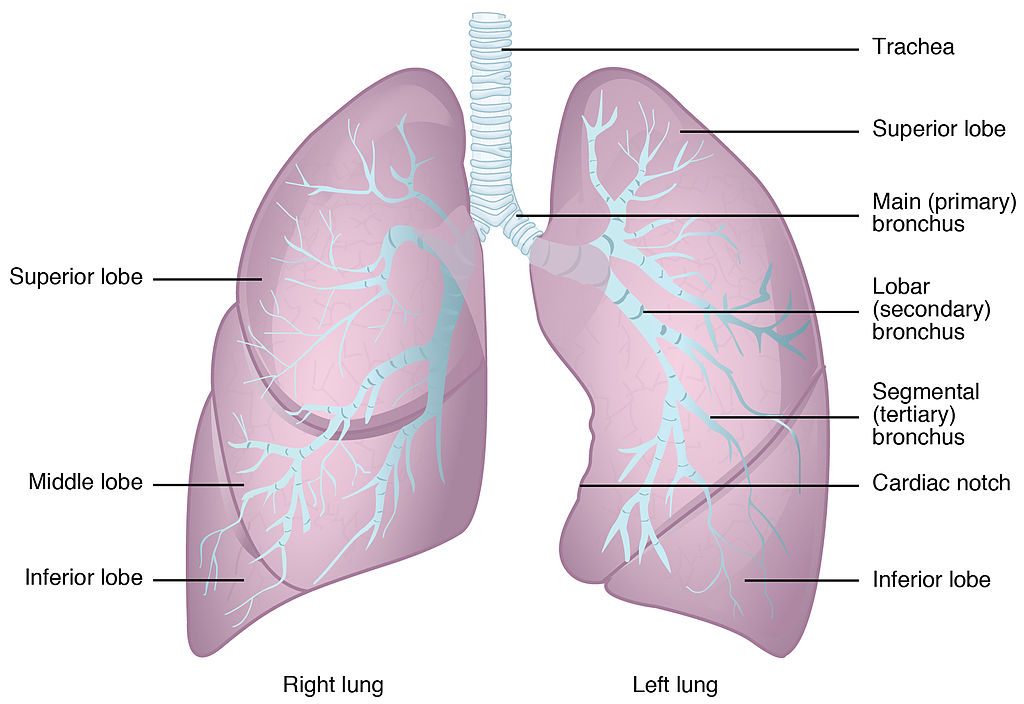
- Bronchioles
Any of the minute branches into which a bronchus divides.
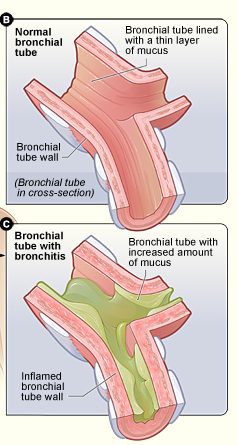
- Bronchitis
Inflammation of the mucous membrane in the bronchial tubes. It typically causes bronchospasm and coughing
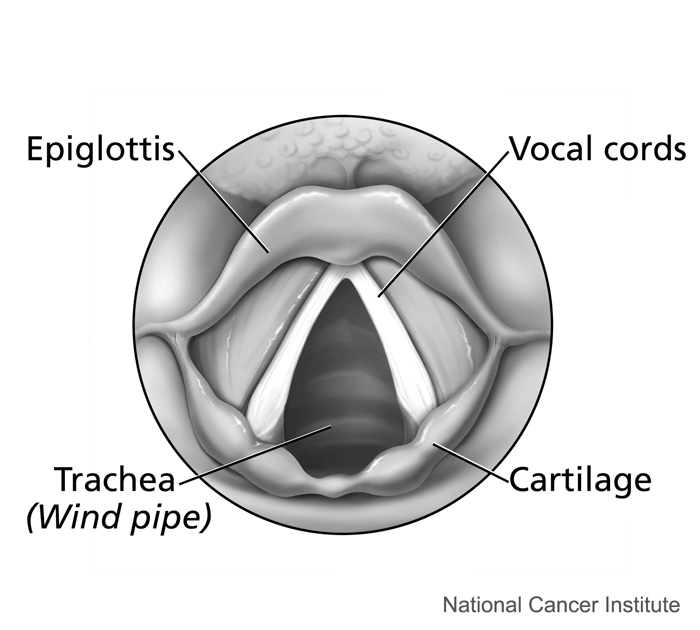
- Bronchus
One of many tubes of various sizes that carry air between the trachea and the alveoli in the lungs.
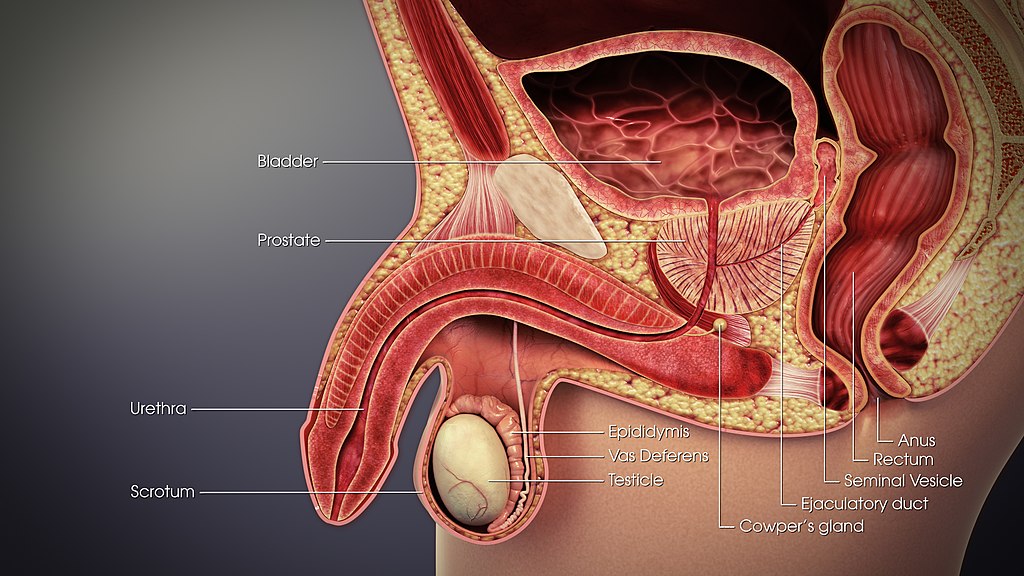
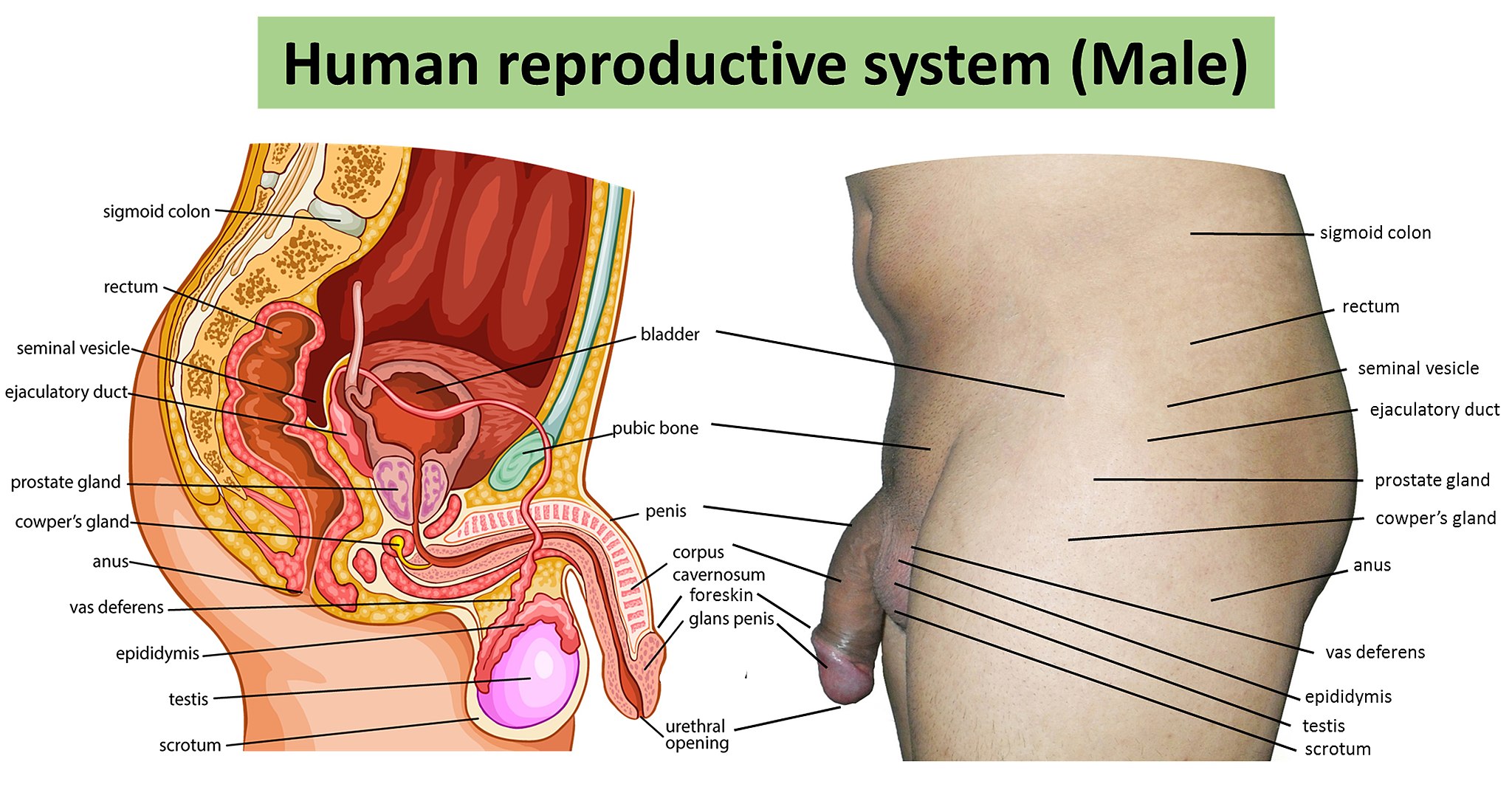
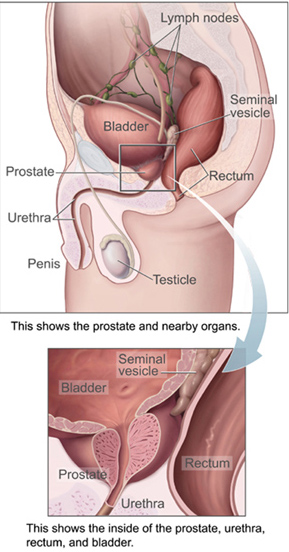
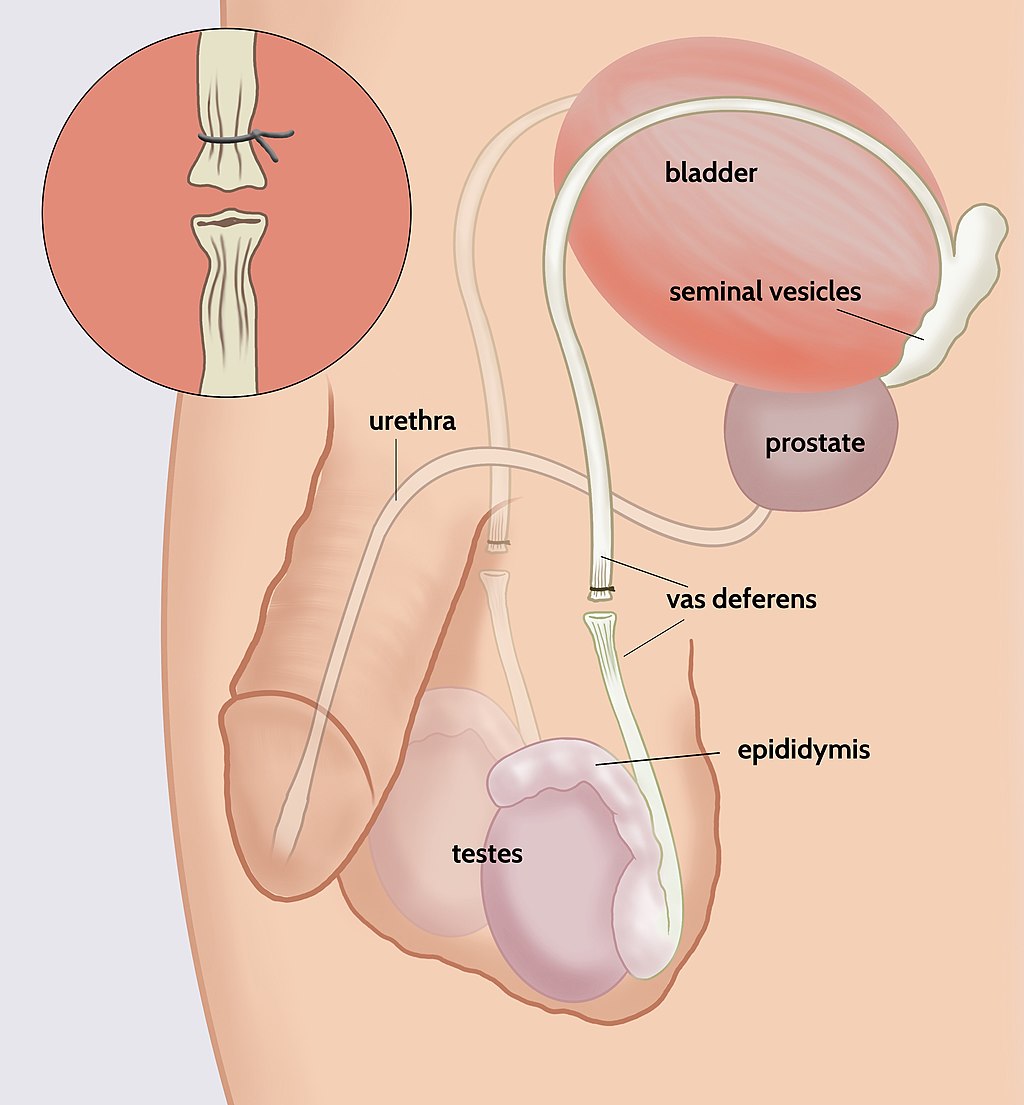
- Bulbourethral gland
One of a pair of glands in the male reproductive system that secretes a fluid to help lubricate the urethra and neutralize any urine it may contain before ejaculation occurs (also called Cowper’s gland).
- Caffeine
A central nervous system stimulant of the methylxanthine class. It is the world's most widely consumed psychoactive drug. Unlike many other psychoactive substances, it is legal and unregulated in nearly all parts of the world. There are several known mechanisms of action to explain the effects of caffeine.
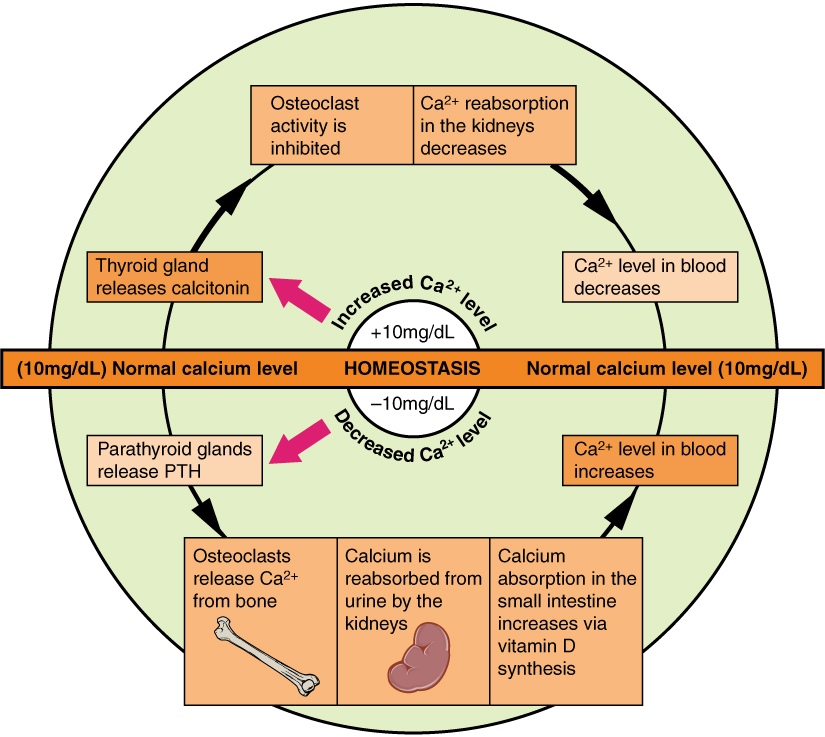
- Calcitonin
A hormone that is produced in humans by the parafollicular cells (commonly known as C-cells) of the thyroid gland. Calcitonin is involved in helping to regulate levels of calcium and phosphate in the blood, opposing the action of parathyroid hormone.
- Calcitriol
The active form of vitamin D, normally made in the kidney. A manufactured form is used to treat kidney disease with low blood calcium, hyperparathyroidism due to kidney disease, low blood calcium due to hypoparathyroidism, osteoporosis, osteomalacia, and familial hypophosphatemia.
- Calcium
A mineral that is necessary for life. In addition to building bones and keeping them healthy, calcium enables our blood to clot, our muscles to contract, and our heart to beat. About 99% of the calcium in our bodies is in our bones and teeth.
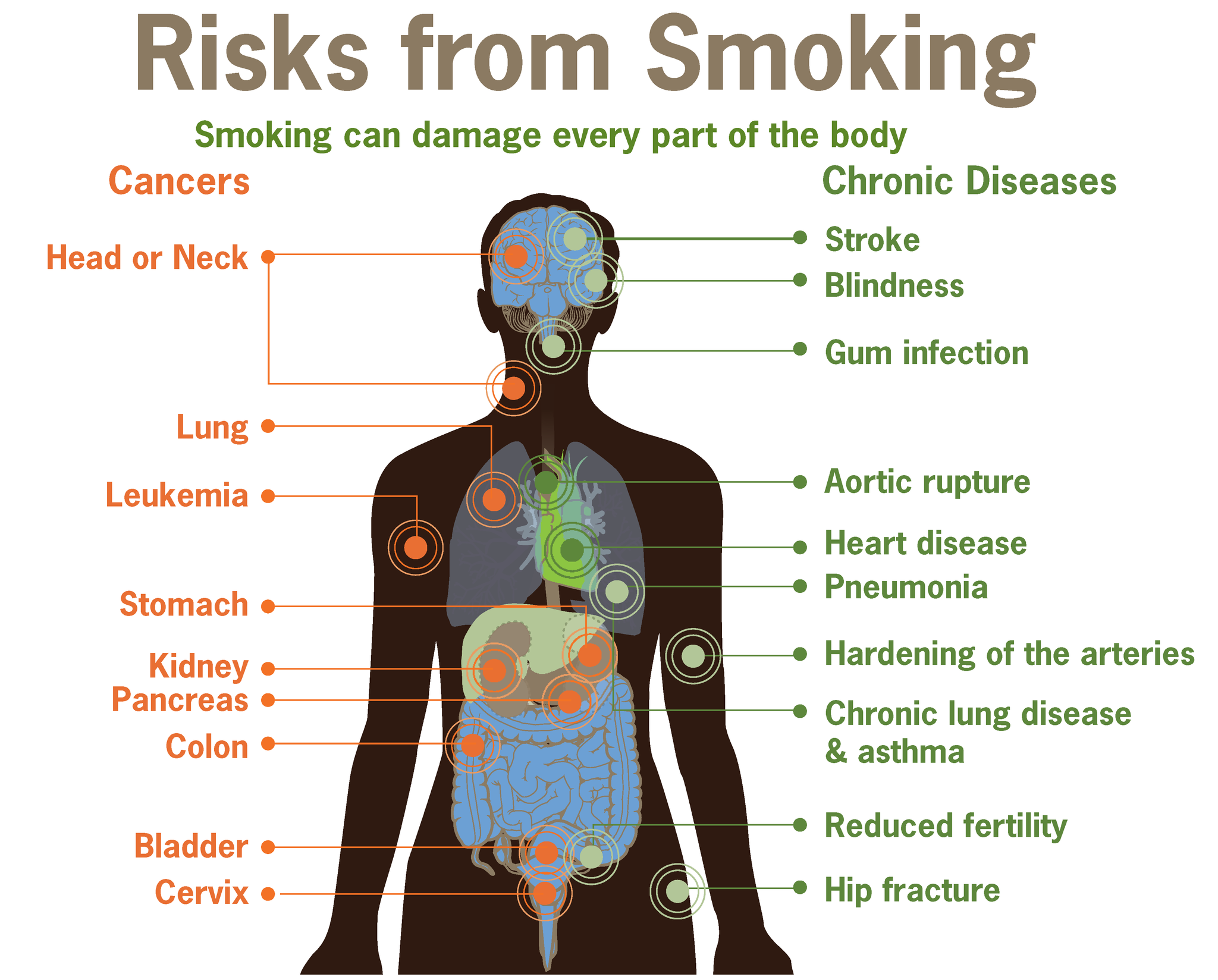
- Cancer
A group of diseases involving abnormal cell growth with the potential to invade or spread to other parts of the body.
- Candidiasis
Infection of the mouth or vagina that is caused by the yeast Candida.
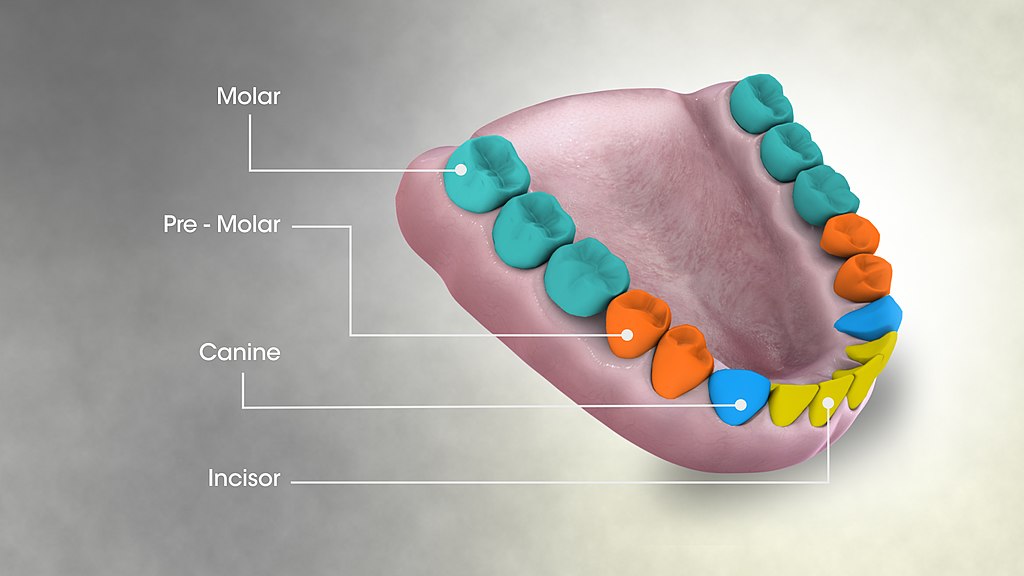
- Canine tooth
One of four pointed teeth on either side of the front teeth that are used for tearing foods.
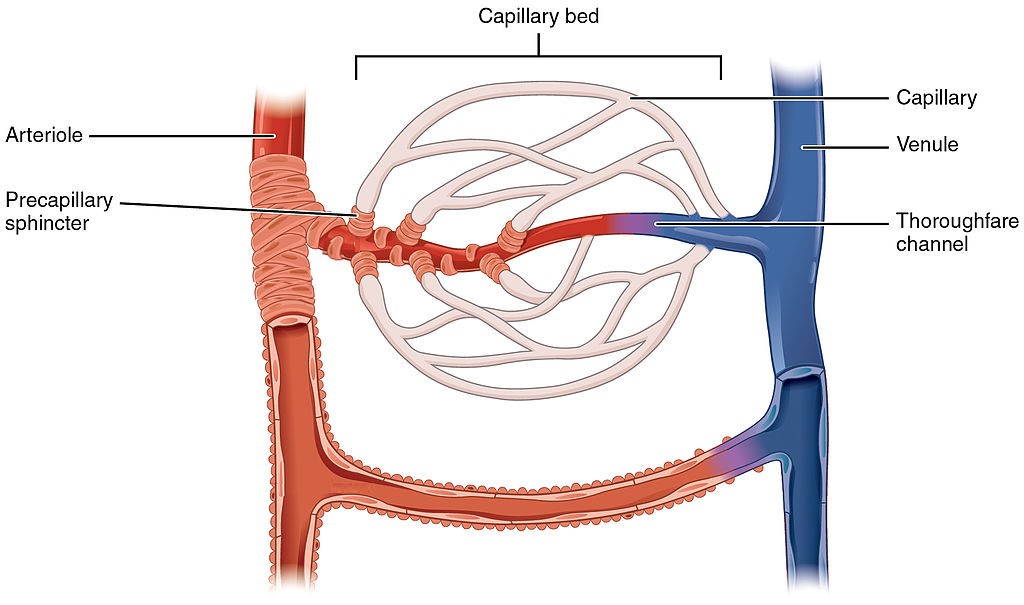
- Capillary
The smallest type of blood vessel that connects arterioles and venules and that transfers substances between blood and tissues.
- Carbohydrates
A biomolecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, usually with a hydrogen–oxygen atom ratio of 2:1. Complex carbohydrates are polymers made from monomers of simple carbohydrates, also termed monosaccharides.
- Carbon monoxide poisoning
Occurs when carbon monoxide builds up in your bloodstream. When too much carbon monoxide is in the air, your body replaces the oxygen in your red blood cells with carbon monoxide. This can lead to serious tissue damage, or even death.
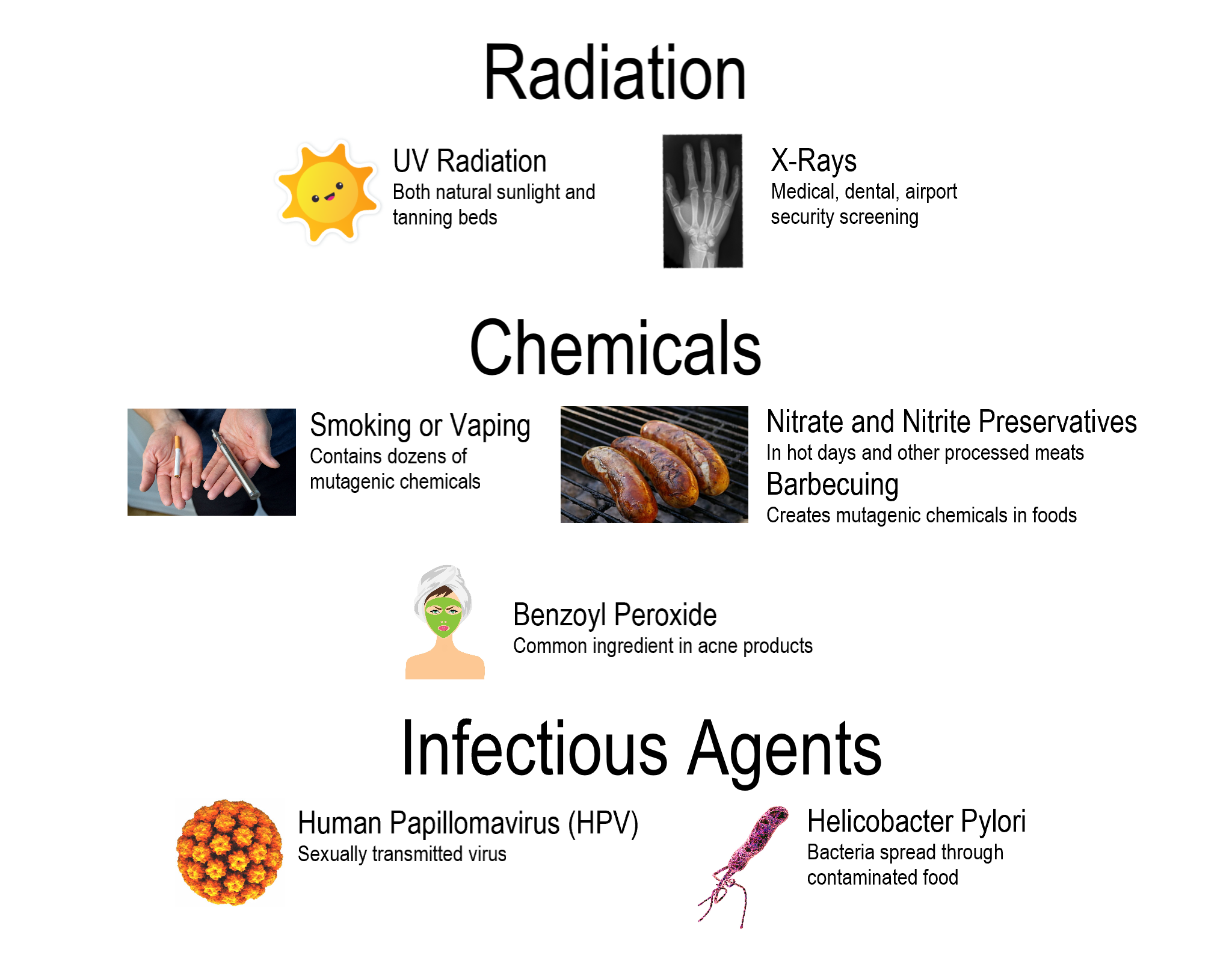
- Carcinogen
A substance capable of causing cancer in living tissue.
- Cardiac arrest
A sudden, sometimes temporary, cessation of function of the heart.
- Cardiac cycle
The performance of the human heart from the ending of one heartbeat to the beginning of the next. It consists of two periods: one during which the heart muscle relaxes and refills with blood, called diastole, following a period of robust contraction and pumping of blood, dubbed systole.
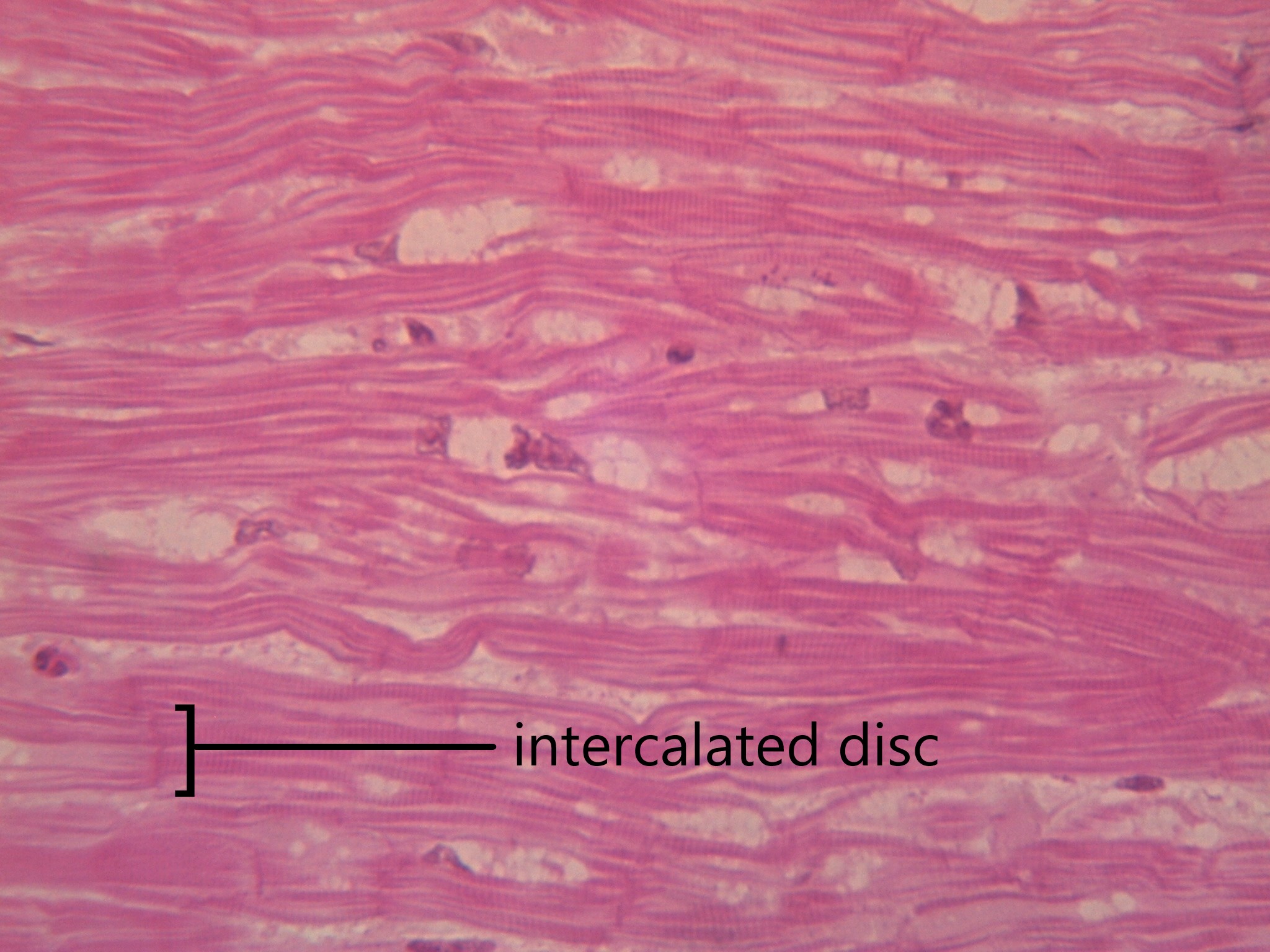
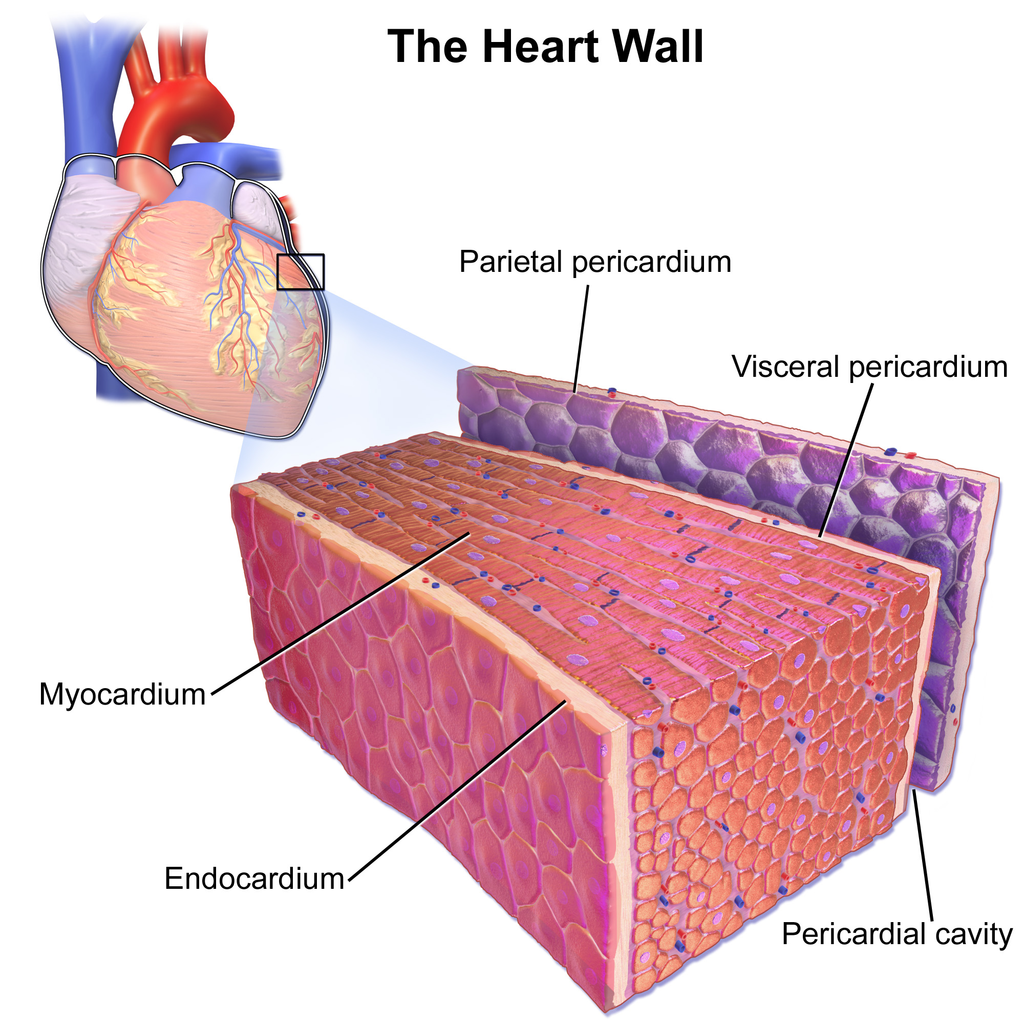
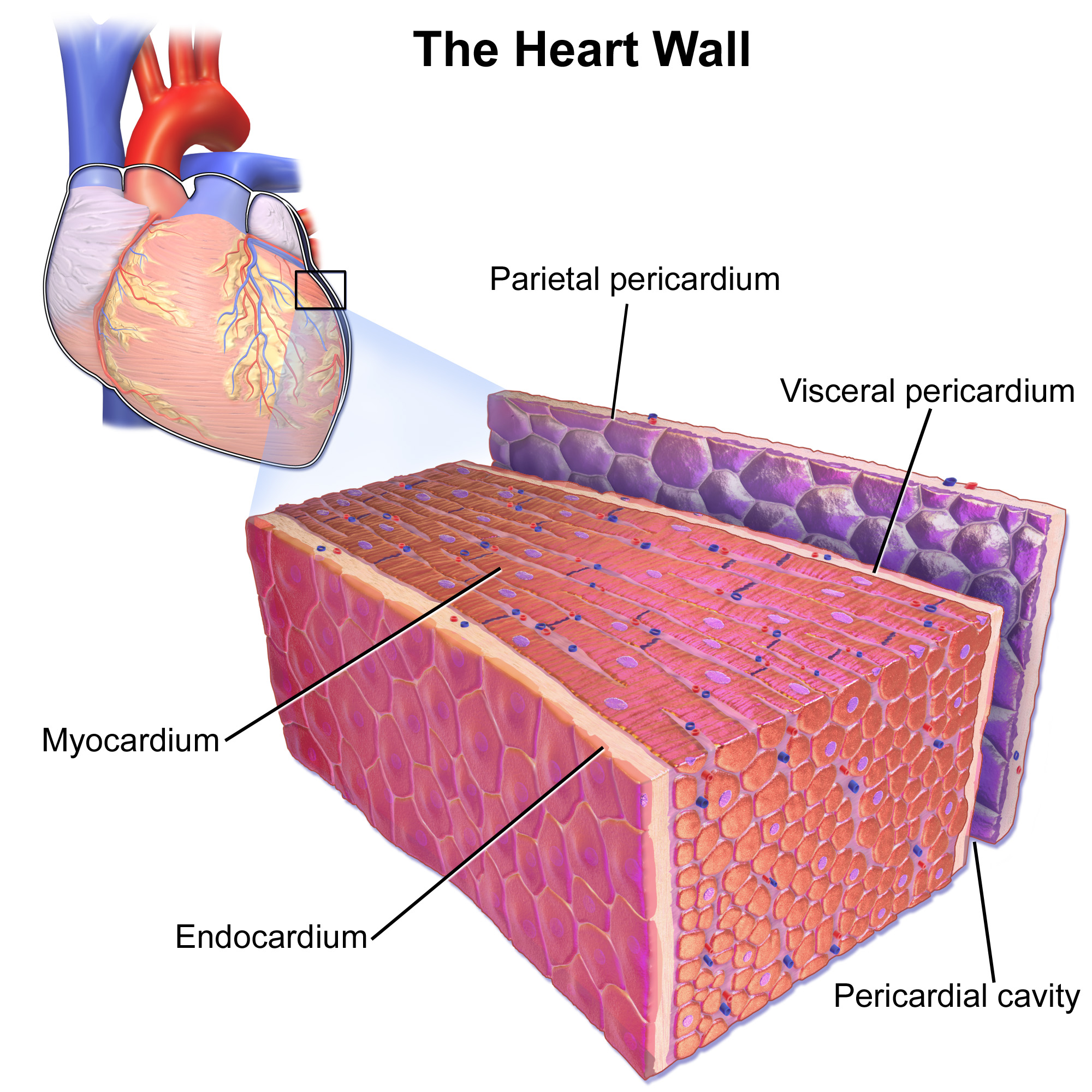
- Cardiac muscle
Involuntary, striated muscle found only in the walls of the heart; also called myocardium.
- Cardiomyocyte
A cardiac muscle cell. The cell is striated, containing thick and thin proteins arranged linearly. These filaments are composed, like other striated muscle cells, largely of actin and myosin. The cell has an abundant supply of mitochondria that supply the energy needed by the cell for regular muscular contraction.
- Cardiovascular disease
A class of diseases that involve the heart or blood vessels.
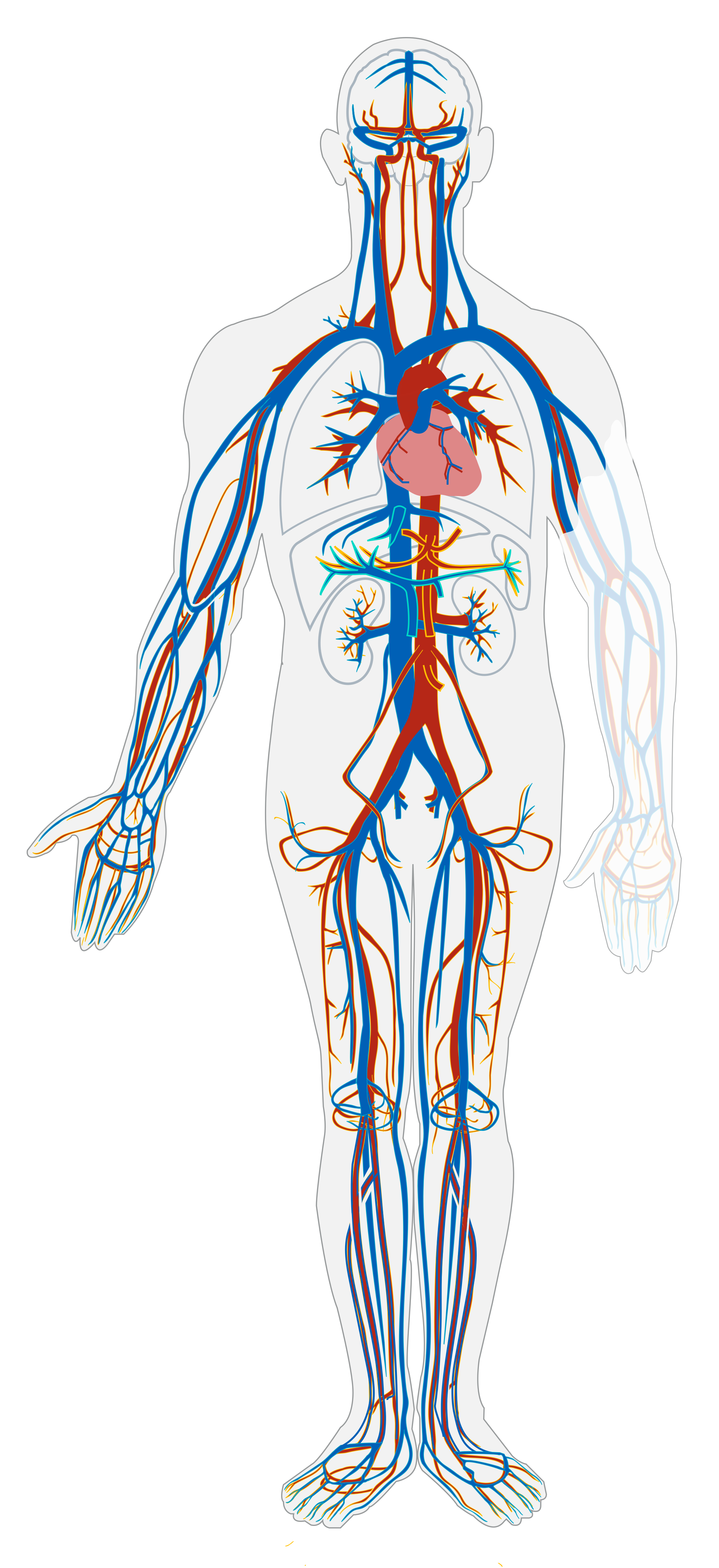
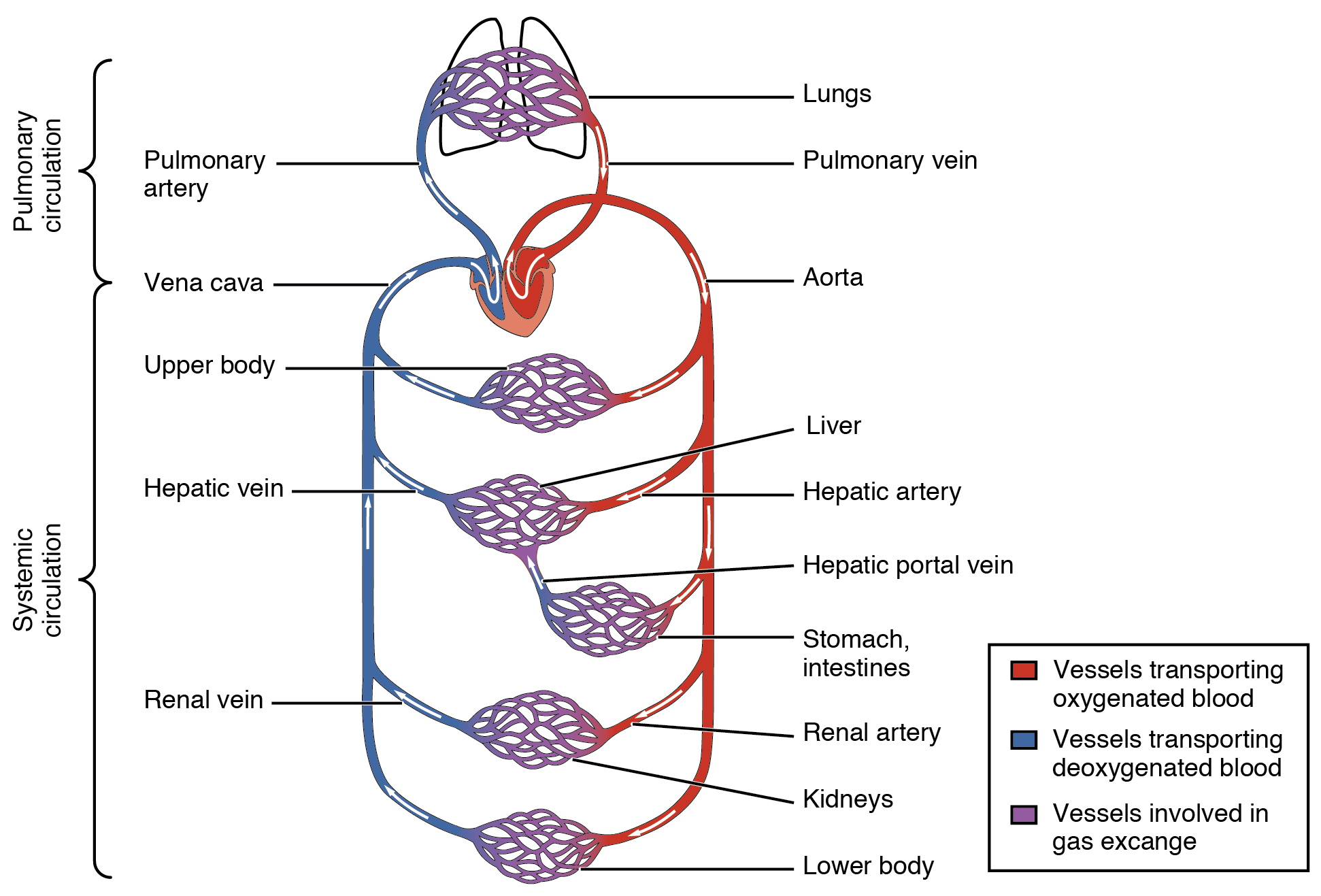
- Cardiovascular system
Refers to the body system consisting of the heart, blood vessels and the blood. Blood contains oxygen and other nutrients which your body needs to survive. The body takes these essential nutrients from the blood.
- Carotene
A pigment in the epidermis that gives skin a yellowish tint, especially in skin with low levels of melanin.
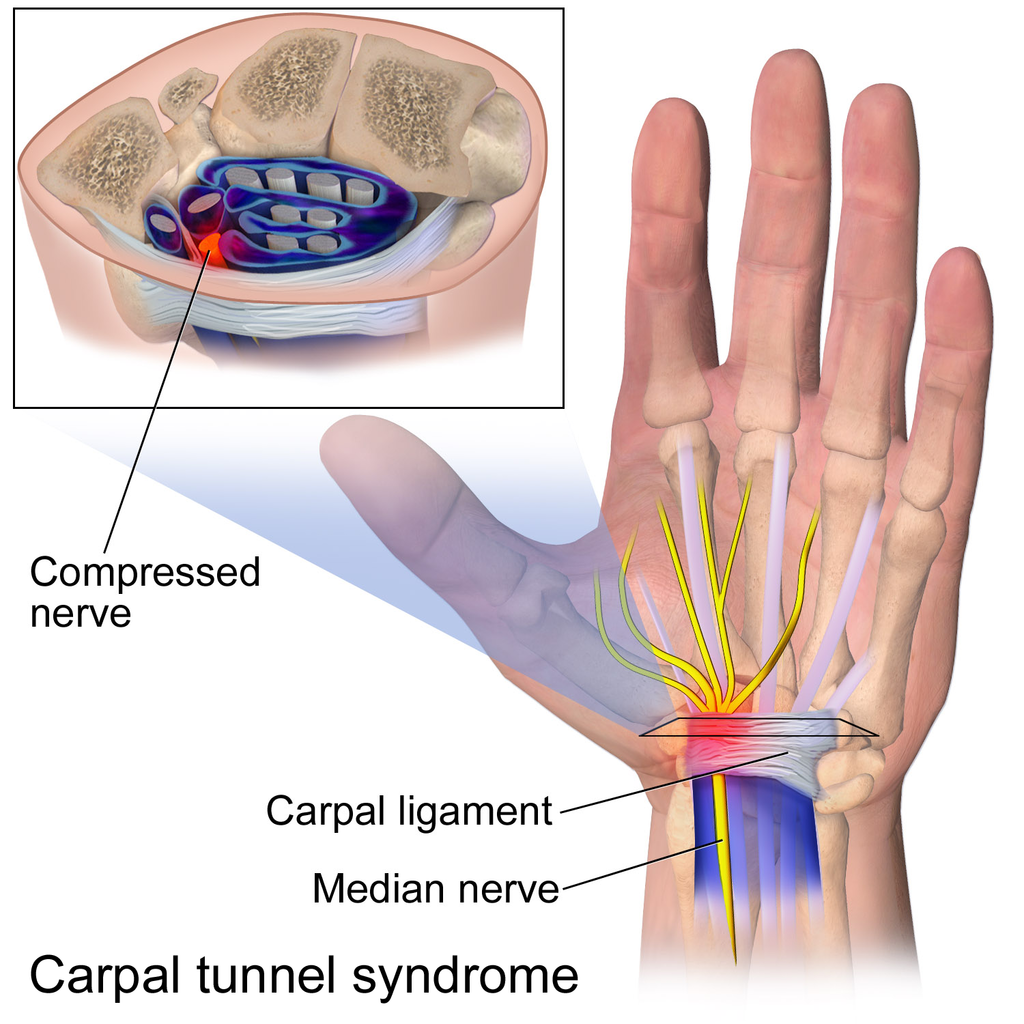
- Carpal tunnel syndrome
A musculoskeletal disorder that occurs when a nerve becomes compressed between carpal bones in the wrist, leading to reduced innervation of the thumb and first two fingers.
- Carrier
A person or other organism that has inherited a recessive allele for a genetic trait or mutation but usually does not display that trait or show symptoms of the disease.
- Carrier proteins
Proteins that carry substances from one side of a biological membrane to the other. Many carrier proteins are found in a cell's membrane, though they may also be found in the membranes of internal organelles such as the mitochondria, chloroplasts, nucleolus, and others.
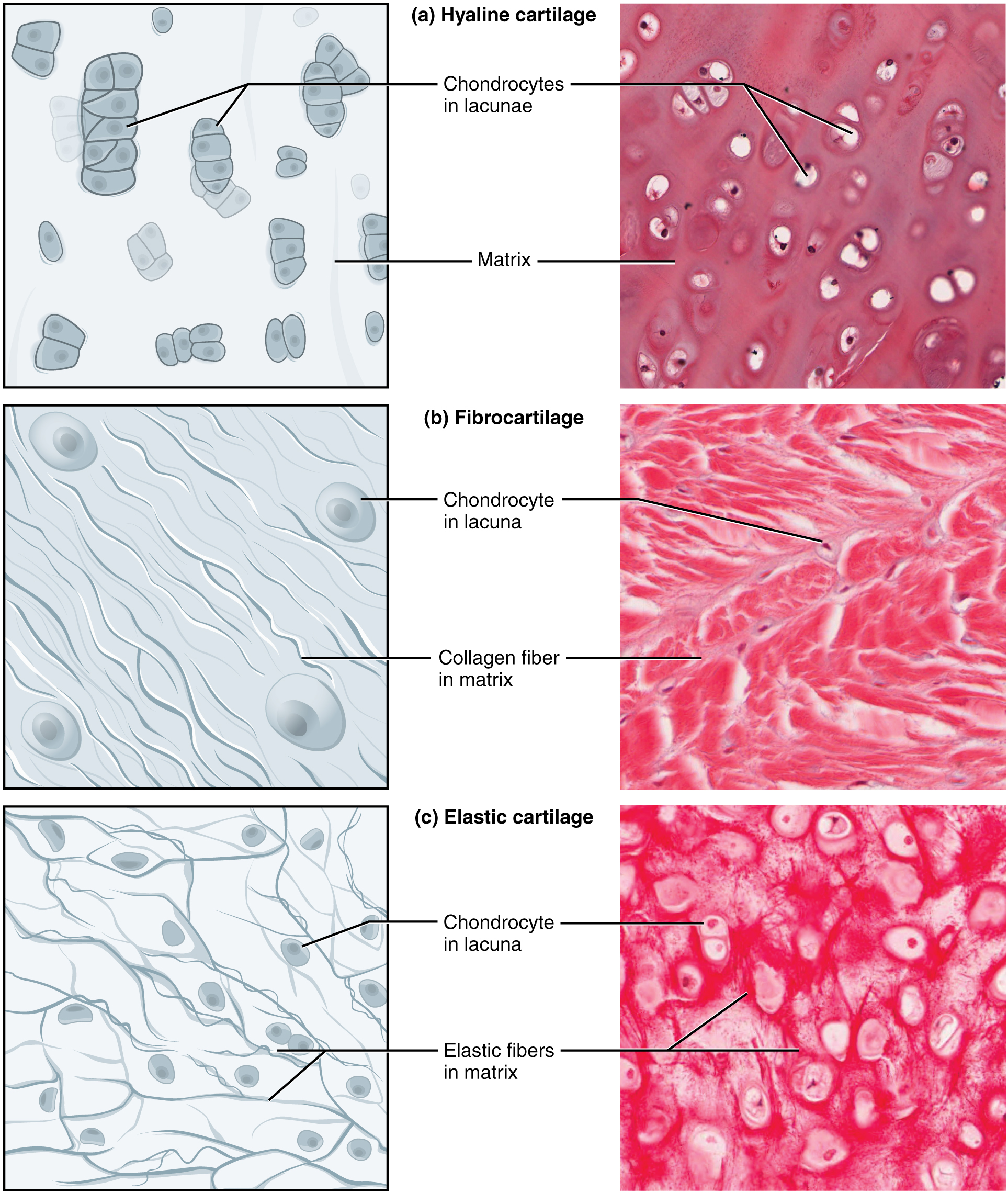
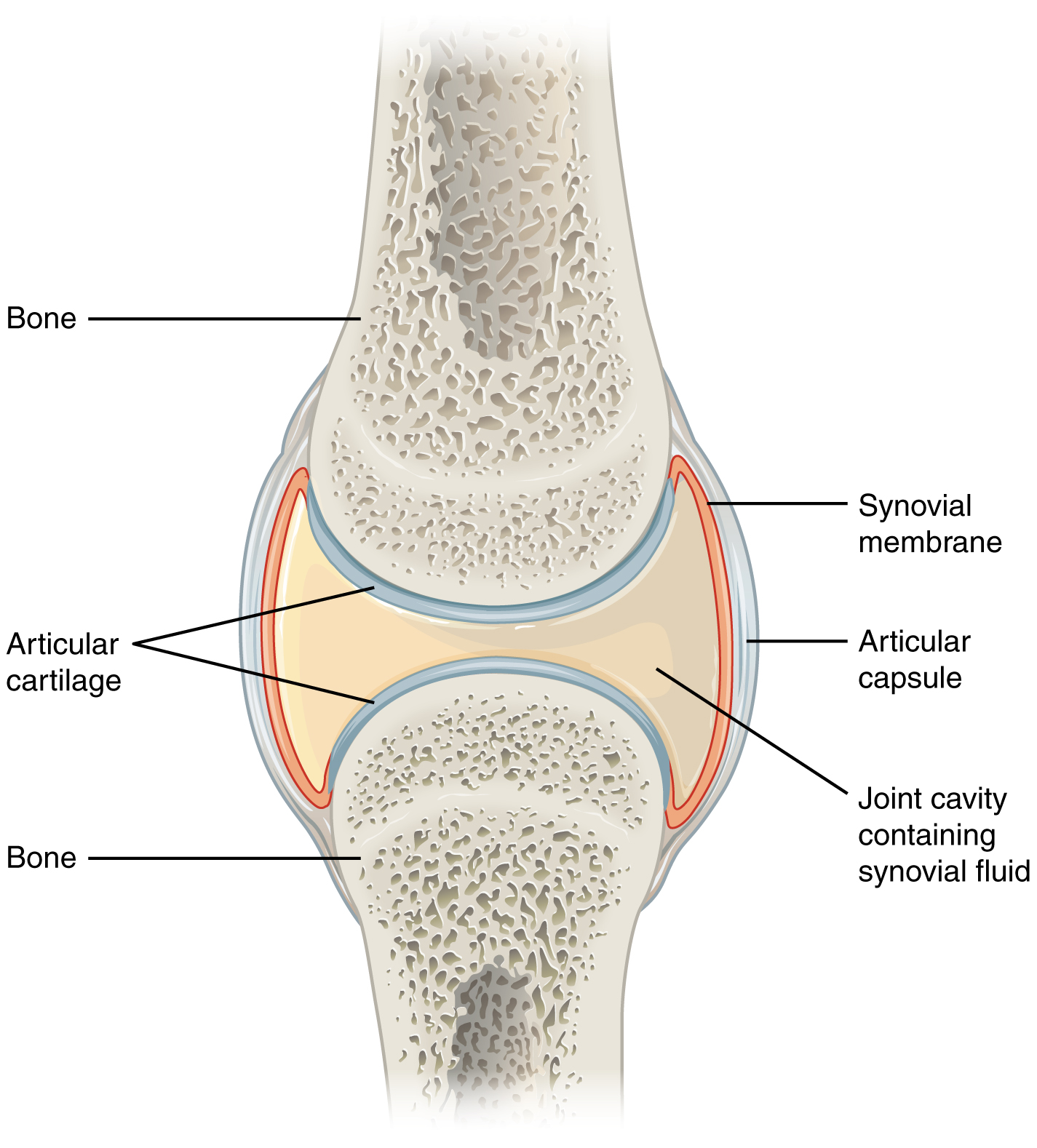
- Cartilage
Supportive connective tissue that provides a smooth surface for the movement of bones at joints. Contains cells called chondrocytes.
- Cartilaginous joint
A partly movable joint in which bones are joined by cartilage.
- Catabolic reaction
A type of metabolic reaction that takes place within a cell in which larger molecules are separated to form smaller molecules.
- Catabolism
The breakdown of larger molecules into smaller ones.
- Catalyst
A substance that increases the rate of a chemical reaction without itself undergoing any permanent chemical change.
- Catecholamine
A class of molecules that includes the non-steroid hormones produced by the medulla of the adrenal gland, such as adrenaline, that stimulate the fight-or-flight response.
- Cecum
A pouch connected to the junction of the small and large intestines.
- Celiac disease
A serious autoimmune disease that occurs in genetically predisposed people where the ingestion of gluten leads to damage in the small intestine. It is estimated to affect 1% of the population worldwide.
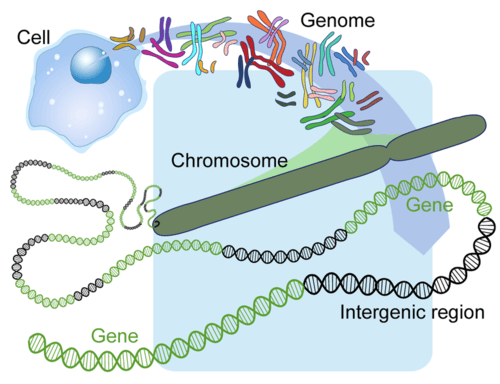
- Cell
The smallest unit of life, consisting of at least a membrane, cytoplasm, and genetic material.
- Cell body
The central part of a neuron that contains the nucleus and other cell organelles.
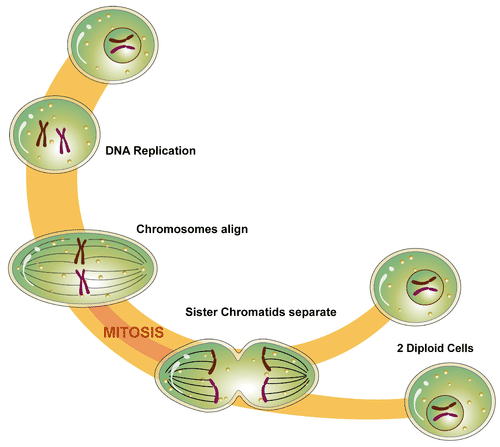
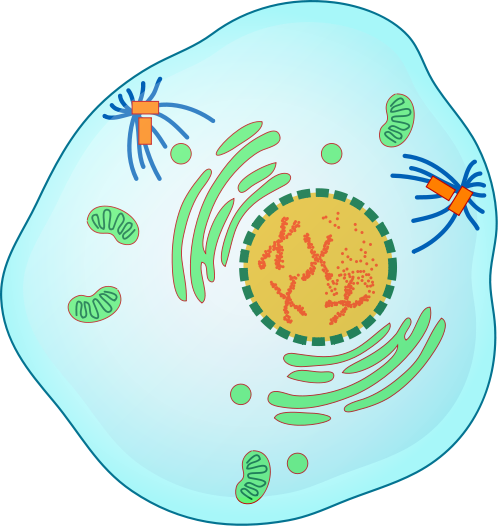
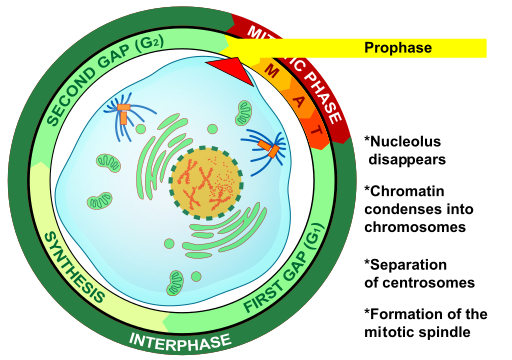
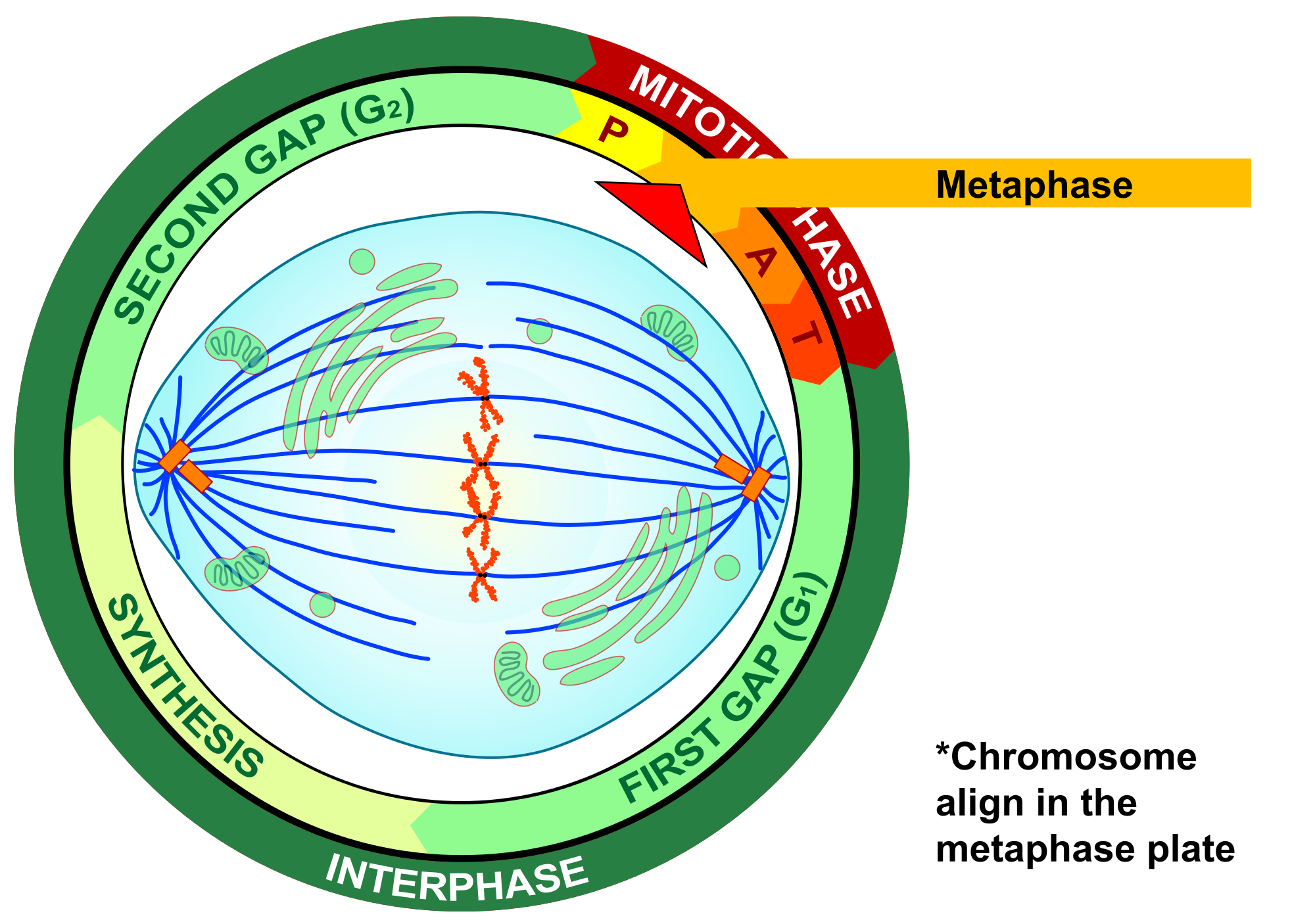
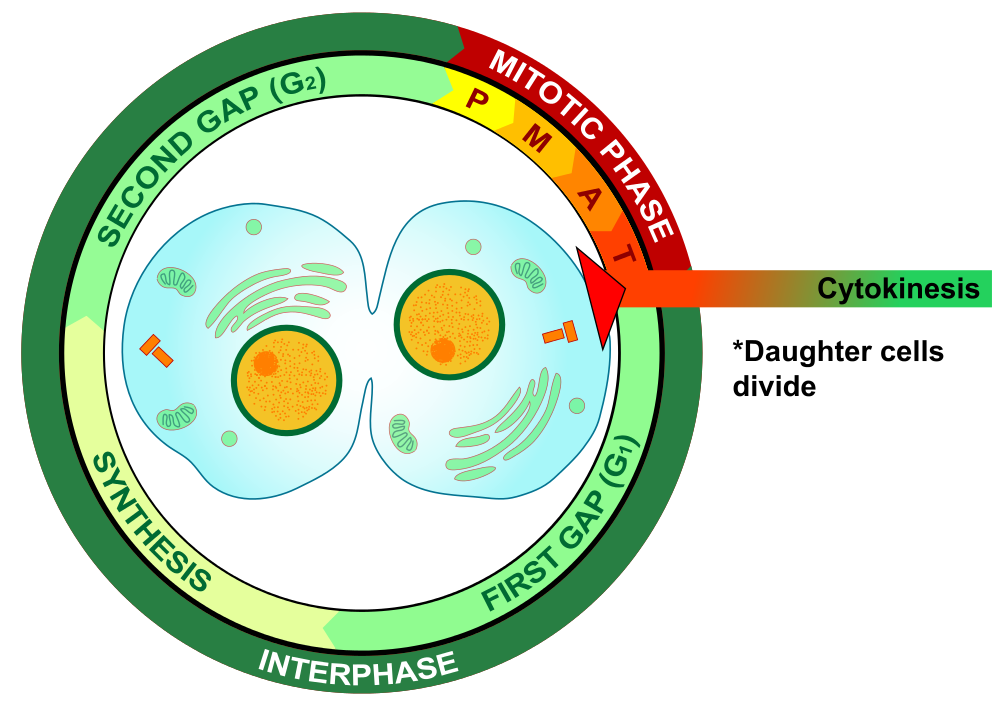
- Cell cycle
A cycle of growth and division that cells go through. It includes interphase (G1, S, and G2) and the mitotic phase.
- Cell division
The process by which a parent cell divides into two or more daughter cells. Cell division usually occurs as part of a larger cell cycle.
- Cell membrane
The semipermeable membrane surrounding the cytoplasm of a cell.
- Cell theory
A historic scientific theory consisting of 3 statements: all living organisms of made of one or more cells, the cell is the basic unit of all living things, and all cells arise from pre-existing cells.
- Cellular respiration
A set of metabolic reactions and processes that take place in the cells of organisms to convert biochemical energy from nutrients into adenosine triphosphate (ATP).
- Cellulose
A substance that makes up most of a plant's cell walls. It is a polymer made up of many linked glucose monomers. Since it is made by all plants, it is probably the most abundant organic compound on Earth.
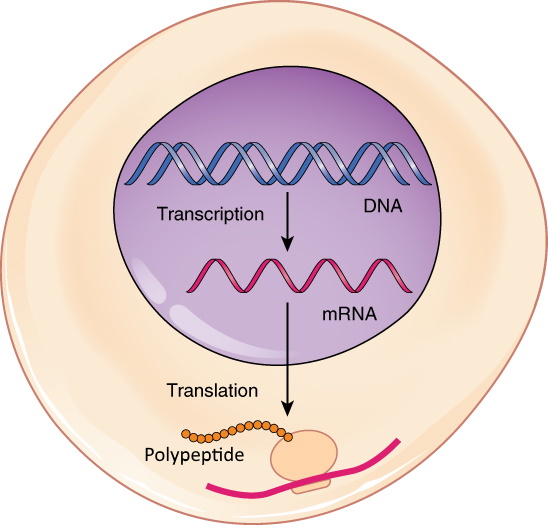
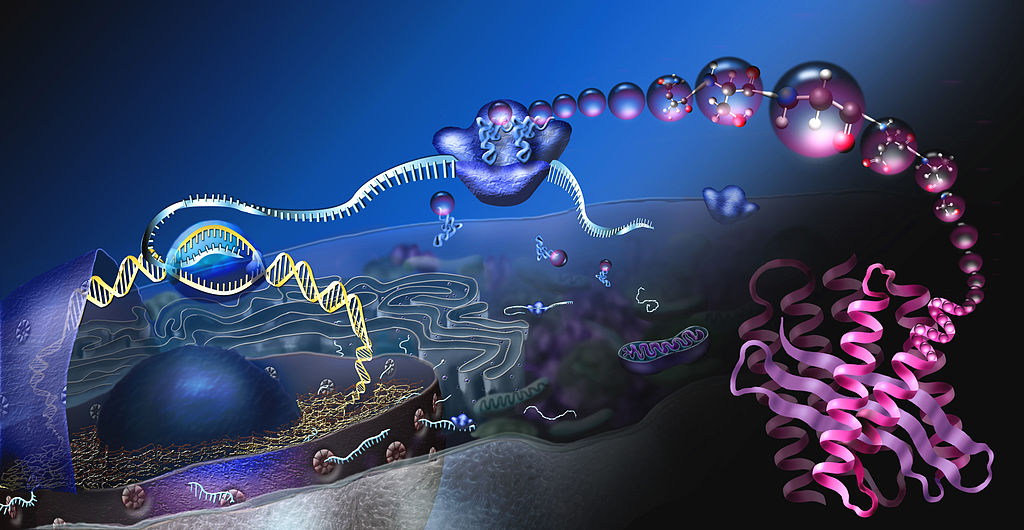
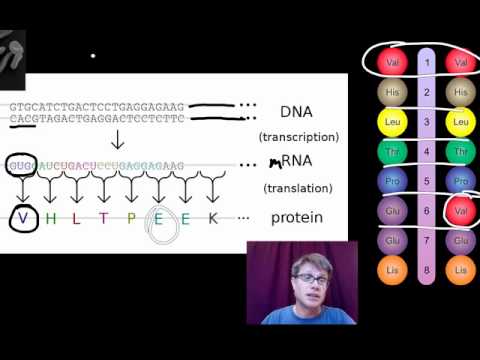
- Central dogma of molecular biology
An explanation of the flow of genetic information within a biological system. It is often stated as "DNA makes RNA and RNA makes protein."
- Central nervous system
One of two main divisions of the nervous system that includes the brain and spinal cord.
- Centriole
A cylindrical organelle composed of microtubules located near the nucleus in animal cells, occurring in pairs and involved in the development of spindle fibers in cell division.
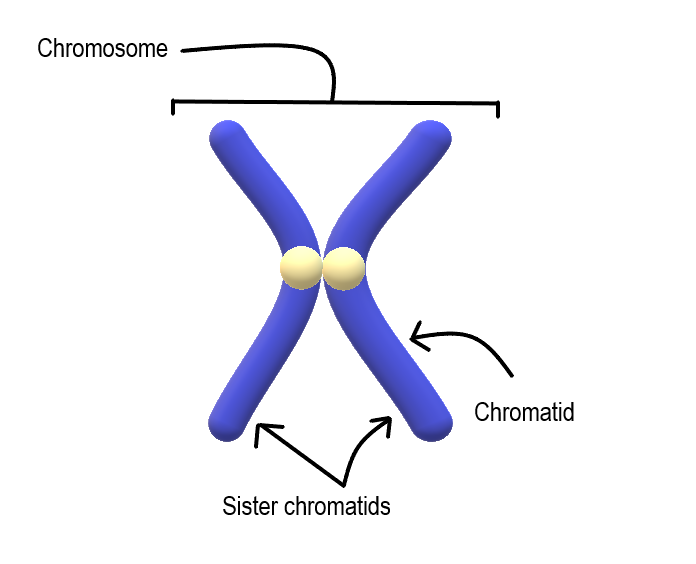
- Centromere
The region in a chromosome that attaches to a spindle fibre at metaphase of mitosis or meiosis.
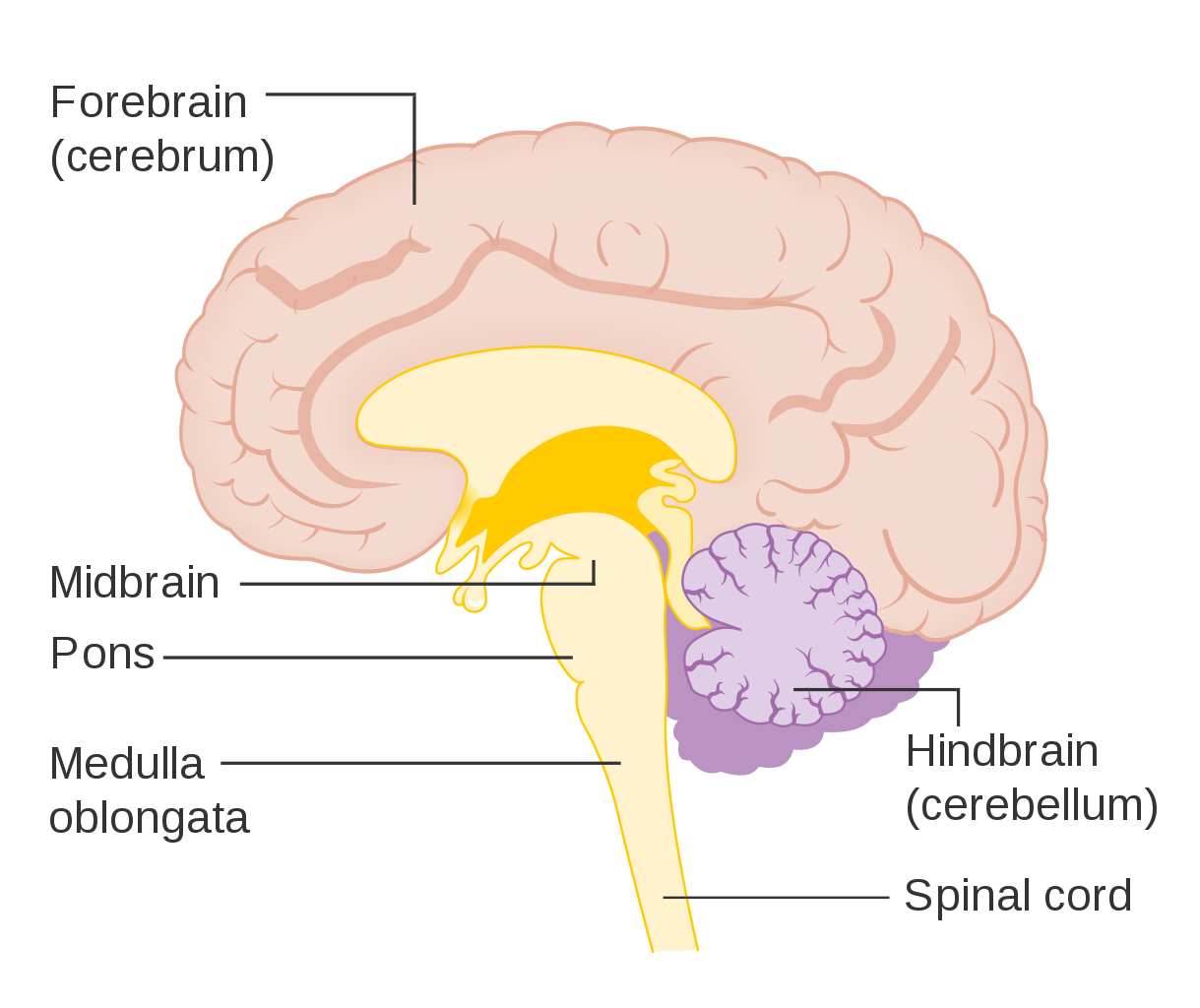
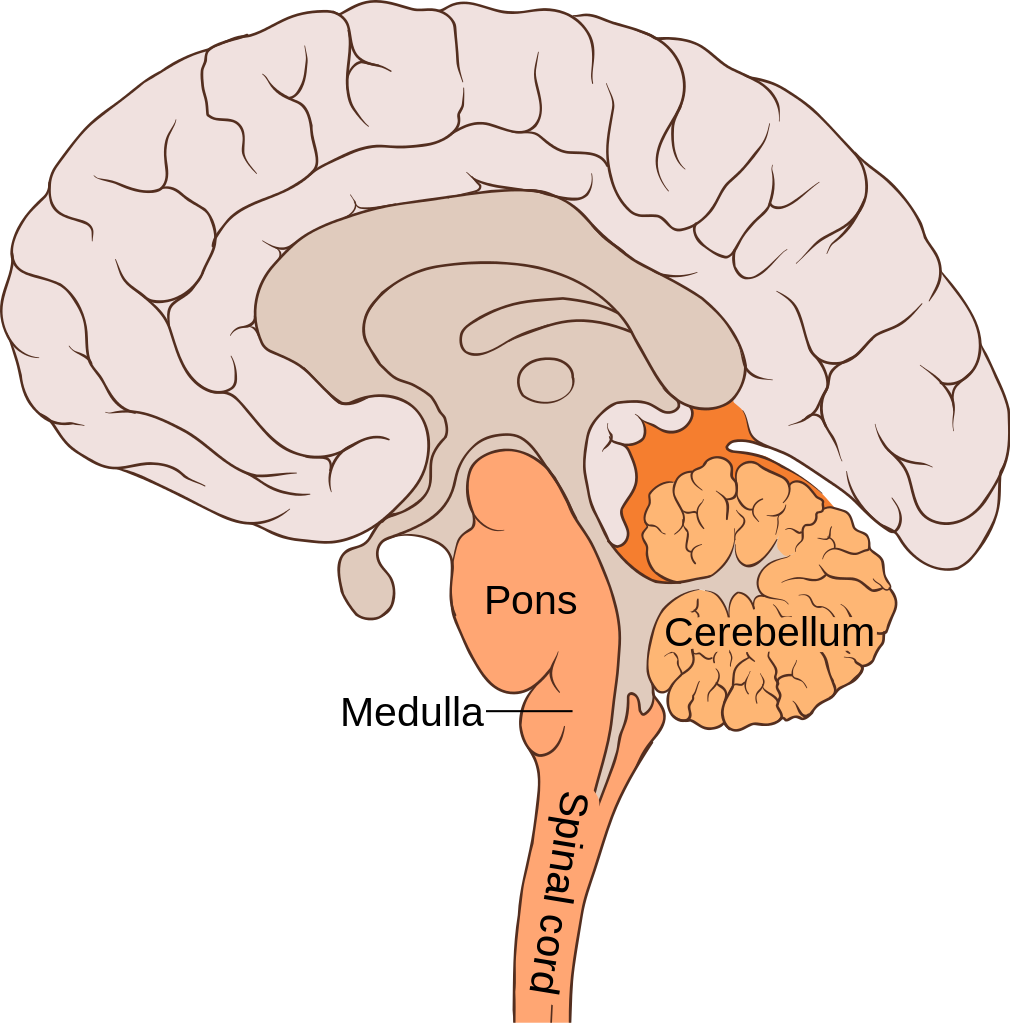
- Cerebellum
The part of the brain below the cerebrum and behind the brain stem that coordinates body movements.
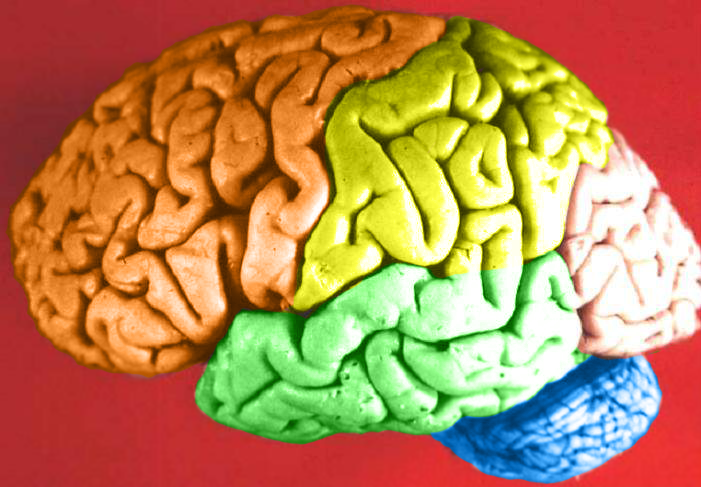
- Cerebral cortex
The highly folded, thin outer layer of the cerebrum where most information processing in the brain takes place.
- Cerebrospinal fluid
Clear fluid produced by the brain that forms a thin layer within the meninges and provides protection and cushioning for the brain and spinal cord.
- Cerebrum
The largest part of the brain that controls conscious functions such as reasoning and sight.
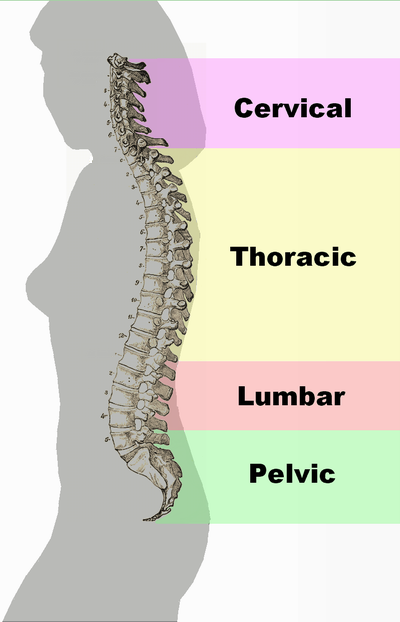
- Cervical
The region of the spinal column containing the vertebrae of the neck, immediately below the skull.
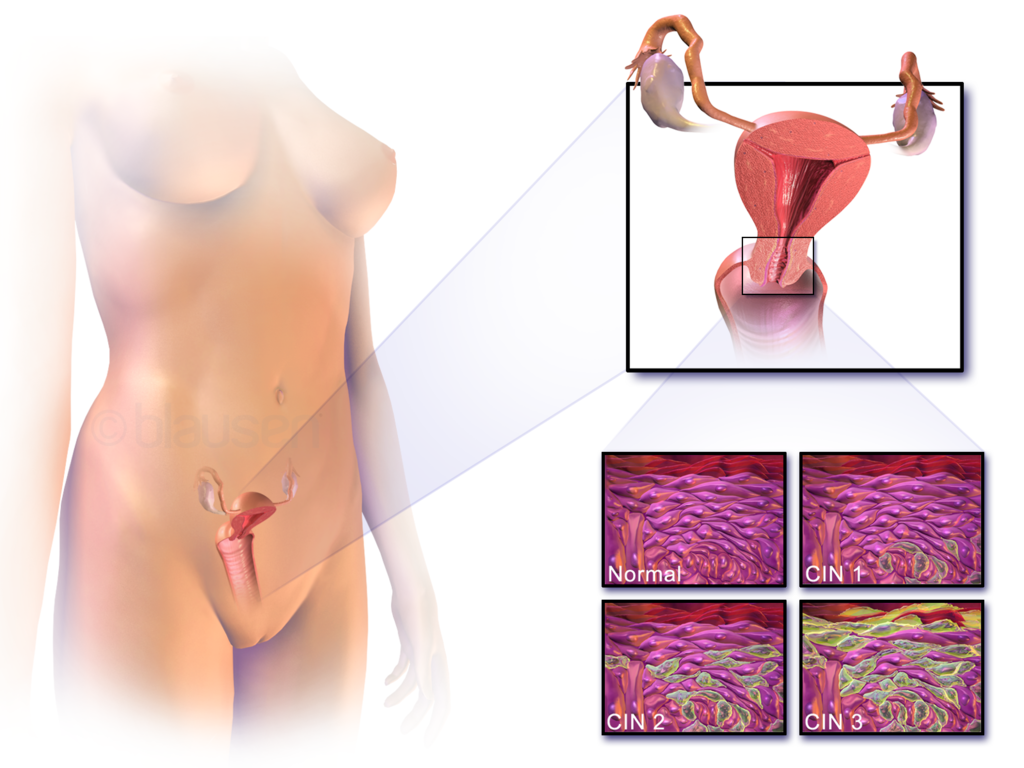
- Cervical cancer
Cancer of the cervix of the uterus, usually caused by infection with human papillomavirus (HPV).
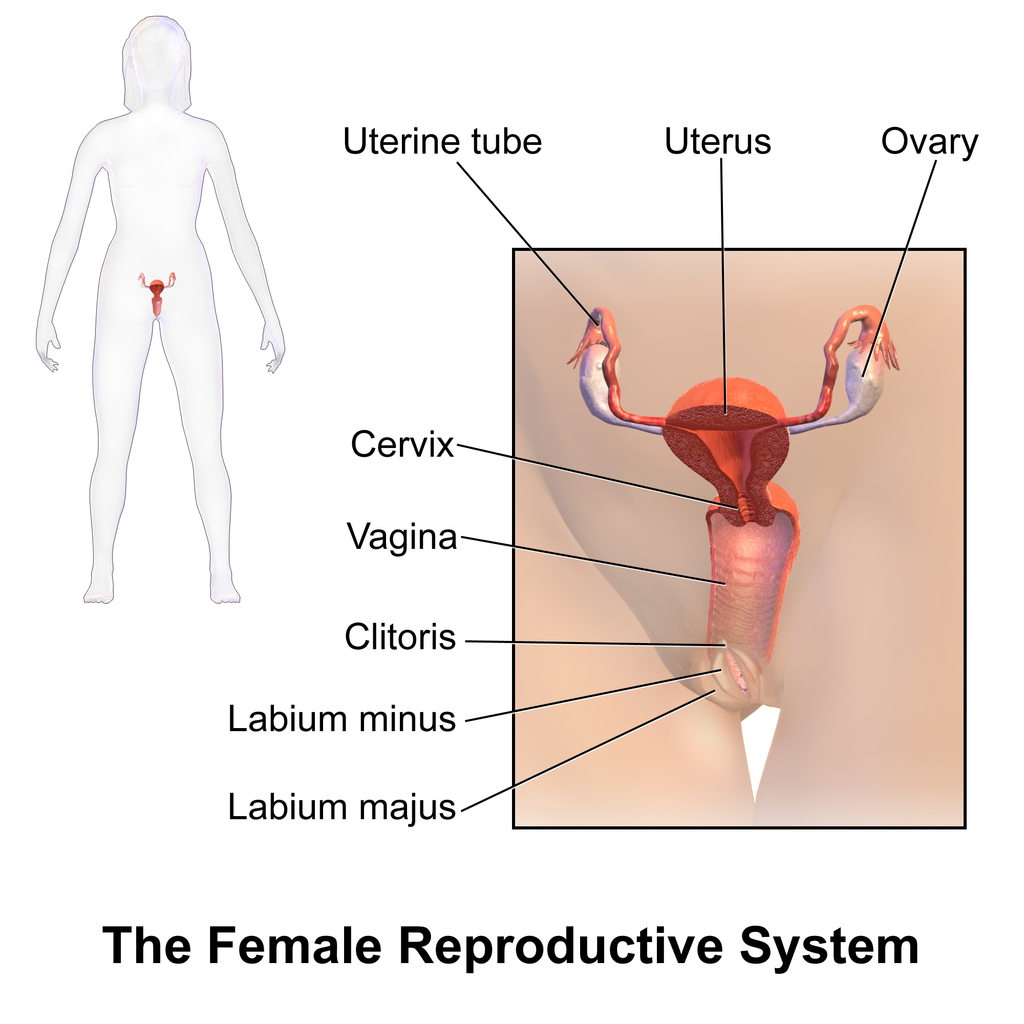
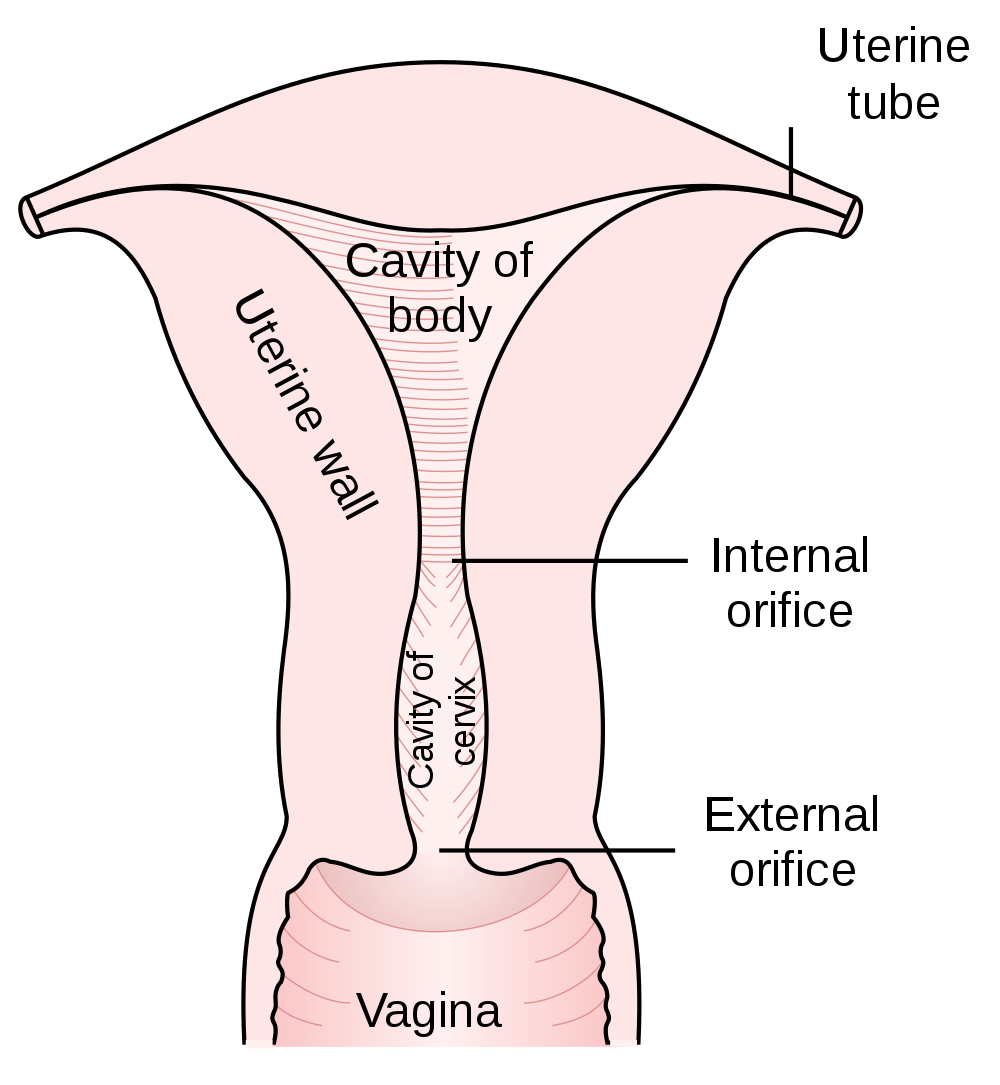
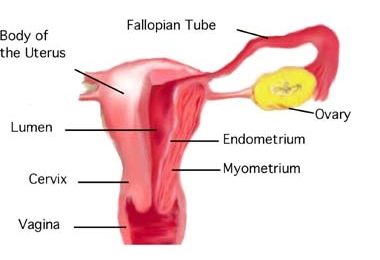
- Cervix
The neck of the uterus that protrudes down into the vagina and through which a canal connects the vagina and uterus.
- Chargaff’s rules
The rules developed by Erwin Chargaff stating that in a double stranded strand of DNA, the amount of adenine is always equal to the amount of thymine, and the amount of guanine is always equal to the amount of cytosine.
- Chemical bond
A chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds.
- Chemical bonds
A lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds.
- Chemical digestion
Chemical breakdown of large, complex food molecules into smaller, simpler nutrient molecules that can be absorbed by blood or lymph. Usually involves a digestive enzyme.
- Chemical equation
An expression that gives the identities and quantities of the substances involved in a reaction. A chemical equation shows the starting compound(s)—the reactants—on the left and the final compound(s)—the products—on the right, separated by an arrow.
- Chemical reaction
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another.
- Chemical substance
A form of matter having constant chemical composition and characteristic properties which cannot be separated into its constituent elements without breaking chemical bonds.
- Chemoreceptor
A type of sensory receptor that responds to presence of chemicals.
- Chemotaxis
The movement of a living structure in response to a chemical signal, (example, as when chemical signals from an egg direct the movement of sperm toward the egg).
- Chemotherapy
The treatment of disease by the use of chemical substances, especially the treatment of cancer by cytotoxic (cell-killing) and other drugs.
- Chitin
A long-chain polymer of linked derivatives of glucose. It is an important structural component in the cell walls of fungi, exoskeletons of insects and crustaceans, and in fish scales.
- Cholesterol
A lipid. Cholesterol and its derivatives are important constituents of cell membranes and precursors of other steroid compounds, but a high proportion in the blood of low-density lipoprotein (which transports cholesterol to the tissues) is associated with an increased risk of coronary heart disease.
- Chondrocyte
A cell which has secreted the matrix of cartilage and become embedded in it.
- Chordae tendineae
Tendon-resembling fibrous cords of connective tissue (sometimes referred to as the heart strings) that connect the papillary muscles to the tricuspid AV valve and the bicuspid AV valve in the heart.
- Chromatin
A mass of genetic material composed of DNA and proteins that condense to form chromosomes during eukaryotic cell division.
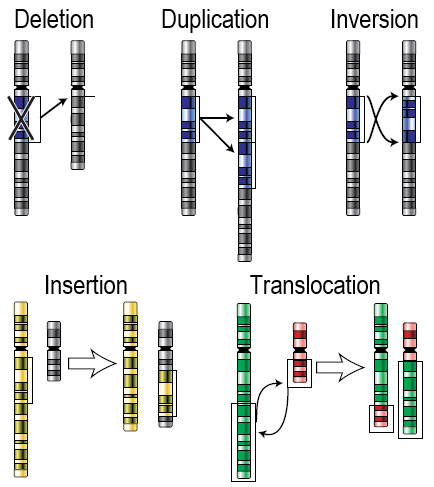
- Chromosomal alteration
A mutation that changes the structure of an individual chromosome, leading to imbalance involving only a part of a chromosome, such as duplication, deletion, or translocation.
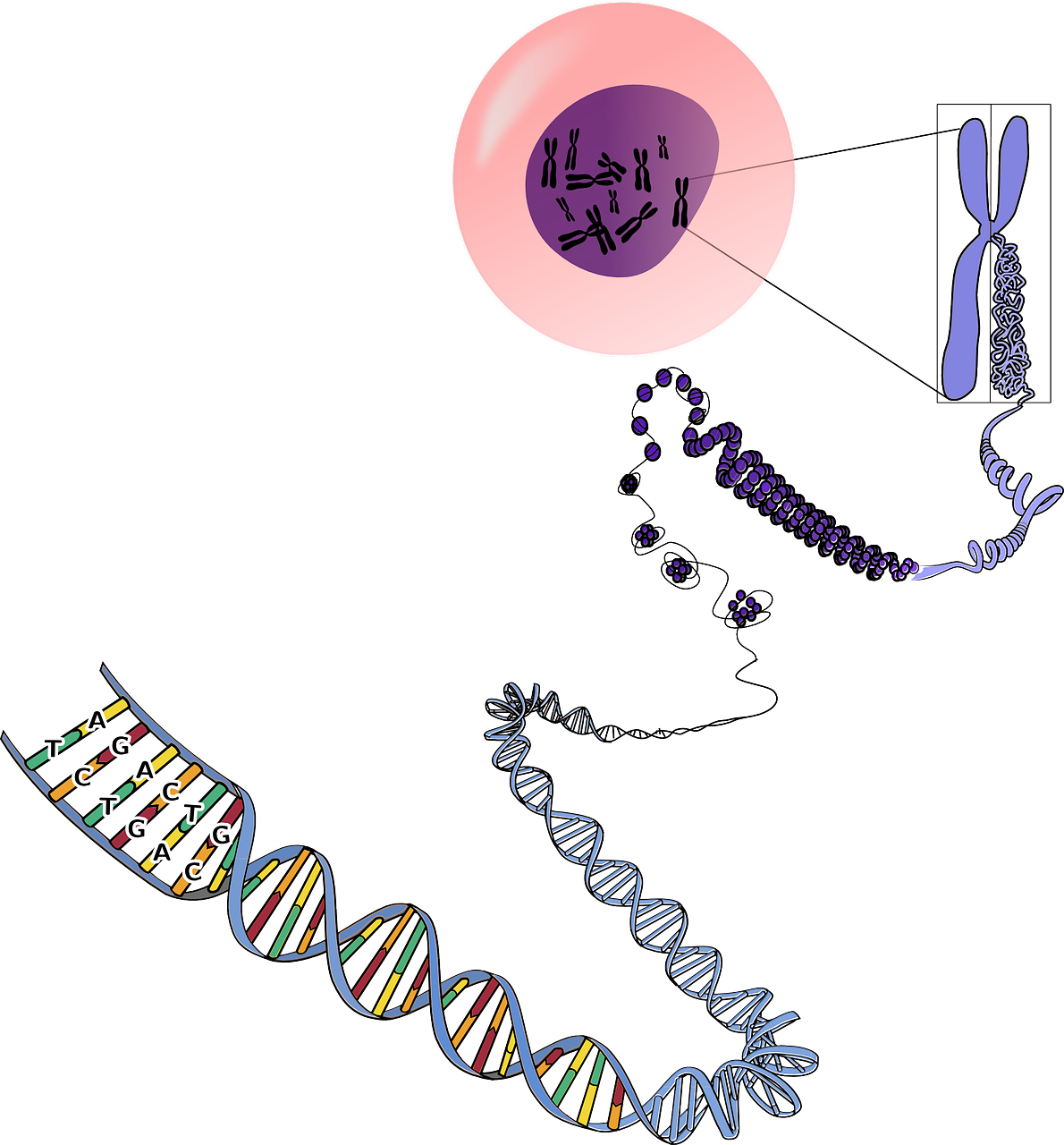
- Chromosome
A threadlike structure of nucleic acids and protein found in the nucleus of most living cells, carrying genetic information in the form of genes.
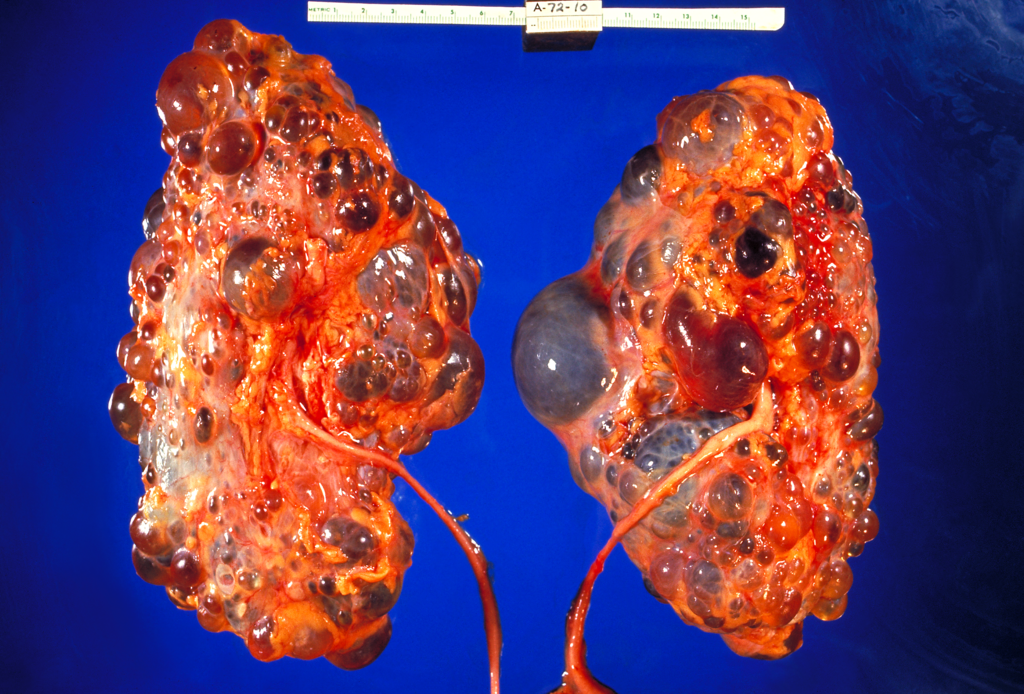
- Chronic kidney disease
Also called chronic kidney failure, describes the gradual loss of kidney function. Your kidneys normally filter wastes and excess fluids from your blood, which are then excreted in your urine.
- Chronic obstructive pulmonary disease
A lung disease characterized by chronic poor airflow, most often following years of tobacco smoking.
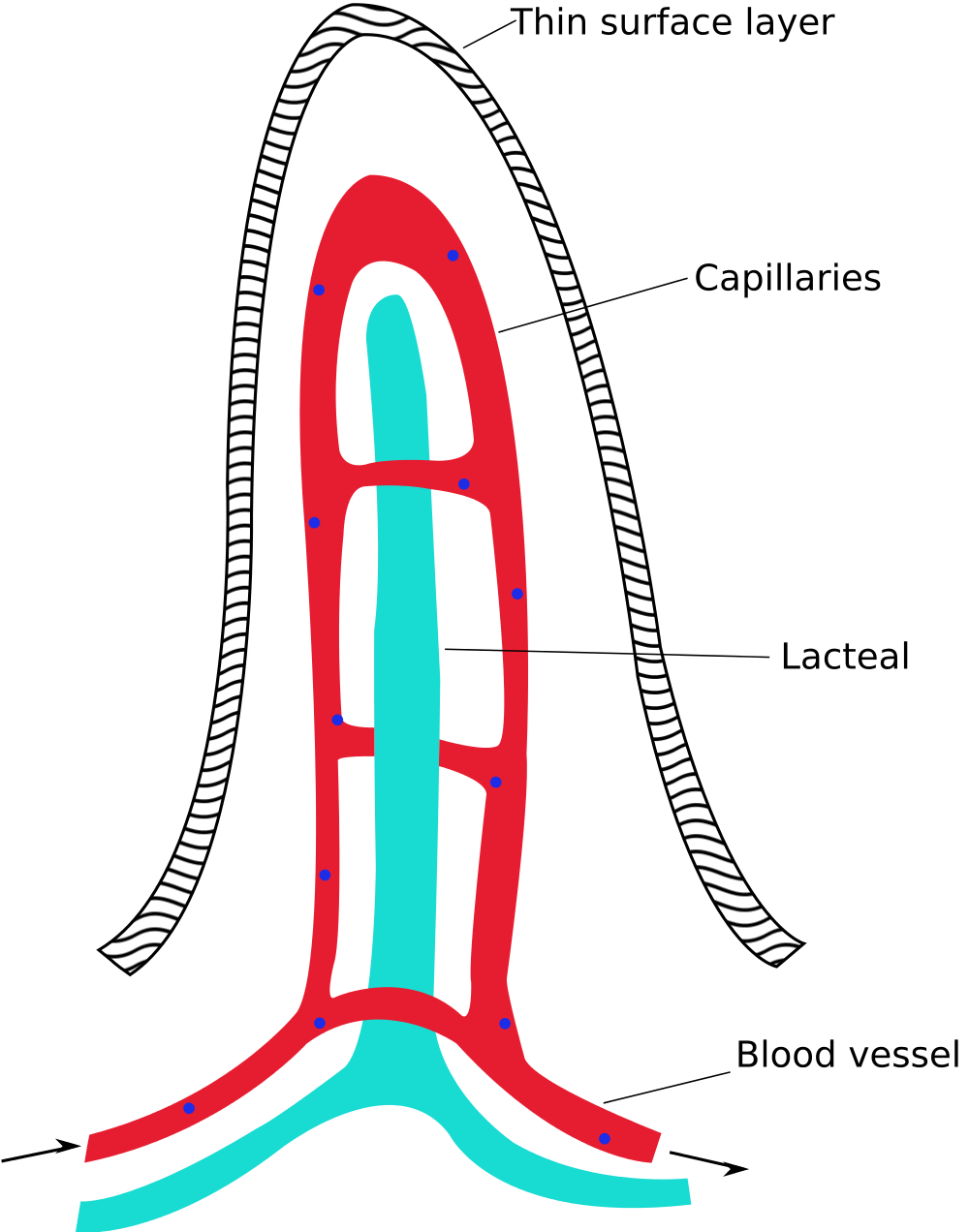
- Chyle
A milky fluid consisting of fat droplets and lymph. It drains from the lacteals of the small intestine into the lymphatic system during digestion.
- Chyme
A thick, semi-liquid mixture that food in the gastrointestinal tract becomes by the time it leaves the stomach.
- Chymotrypsin
A digestive enzyme which breaks down proteins in the small intestine. It is secreted by the pancreas and converted into an active form by trypsin.
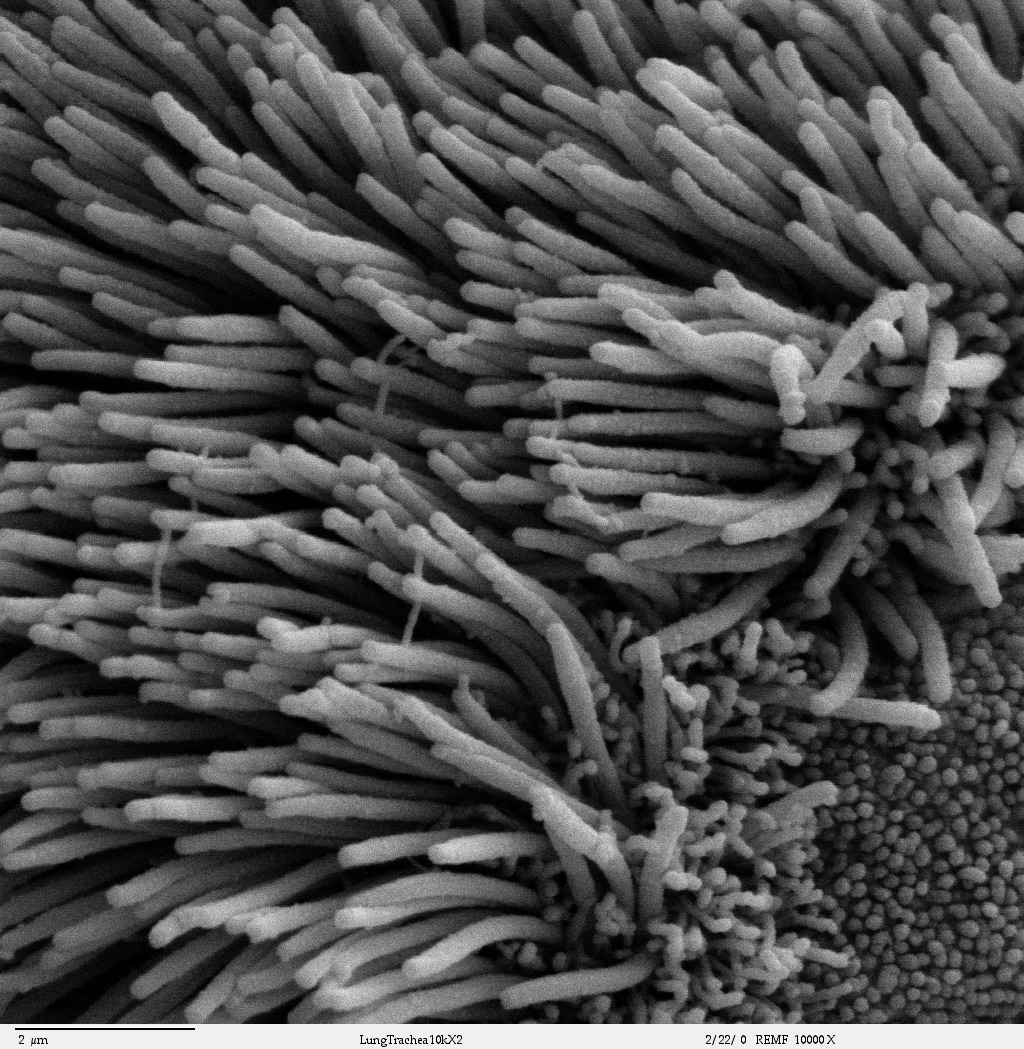
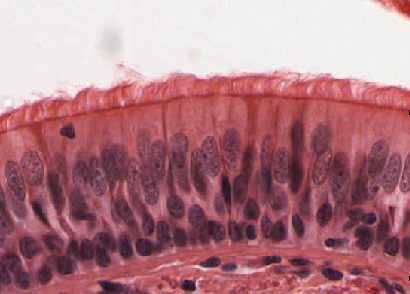
- Cilia
Tiny hairlike organelles, identical in structure to flagella, that line the surfaces of certain cells and beat in rhythmic waves, providing locomotion to ciliate protozoans and moving liquids along internal epithelial tissue in animals.
- Classify
To arrange a group of living things into classes or categories according to shared qualities or characteristics.
- Climate
The long-term average of weather, typically averaged over a period of 30 years. Some of the meteorological variables that are commonly measured are temperature, humidity, atmospheric pressure, wind, and precipitation.
- Cline
A measurable gradient in a single character (or biological trait) of a species across its geographical range.
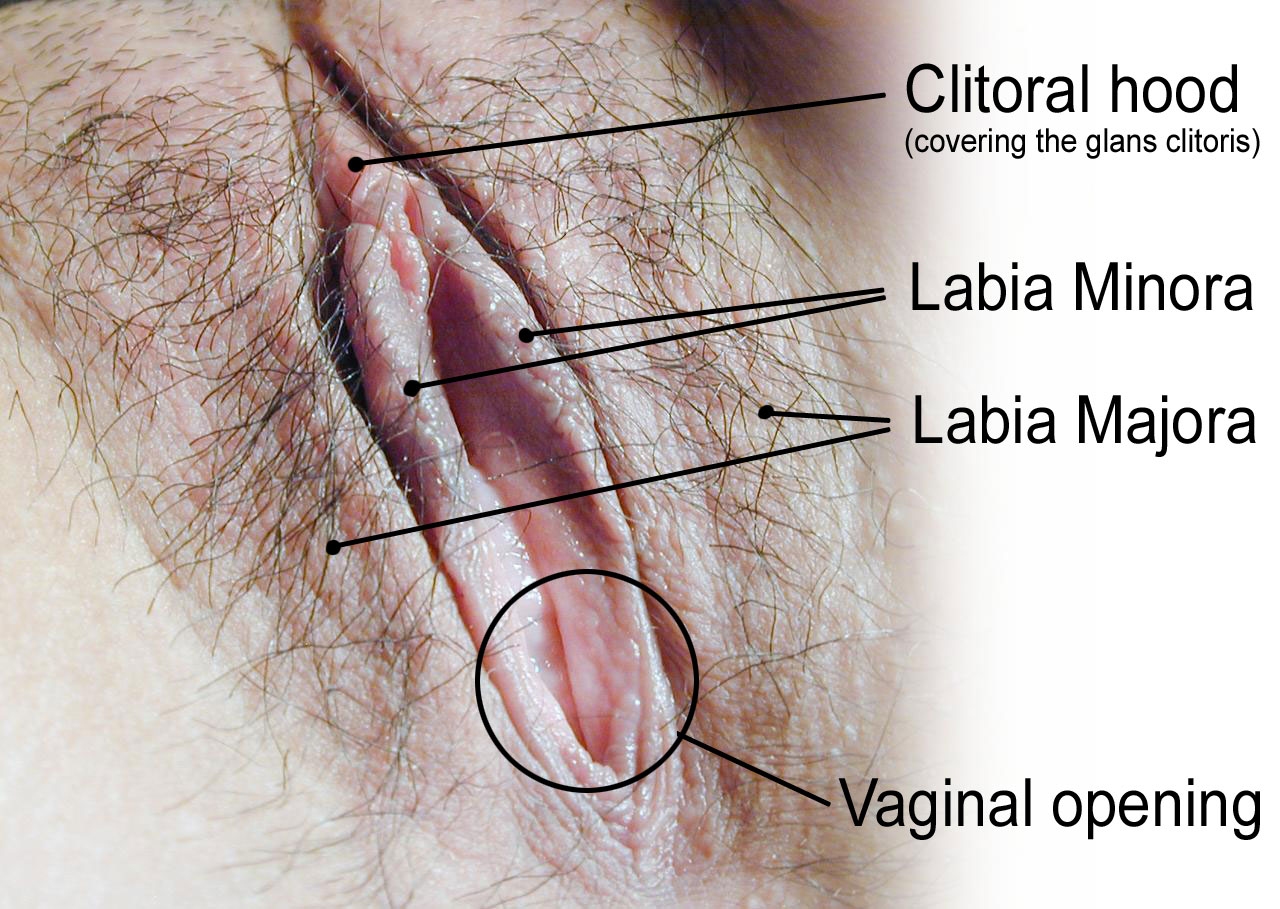
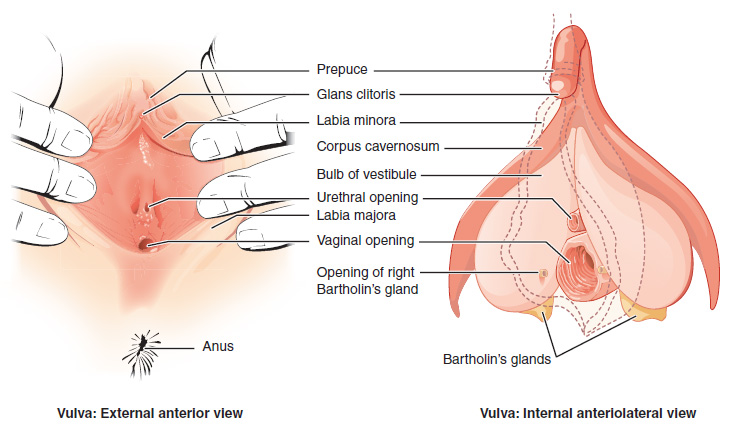
- Clitoris
The small, sensitive external female organ that is part of the vulva and may lead to sexual arousal and/or orgasm when stimulated.
- Coagulation
The process by which blood changes from a liquid to a gel to form a blood clot
- Coccygeal
Relating to the coccyx. The coccyx, also known as the tailbone, is a small, triangular bone resembling a shortened tail located at the bottom of the spine.The vertebrae may be fused together to form a single bone; however, in some cases, the first vertebra is separate from the others.
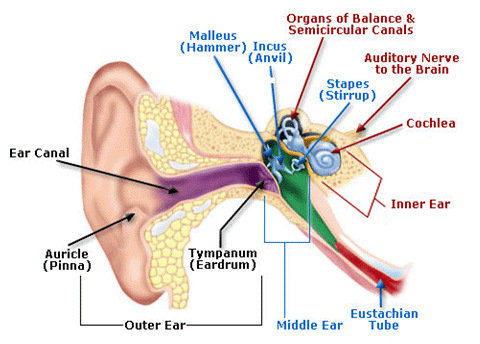
- Cochlea
A coiled, fluid-filled tube in the inner ear that changes mechanical sound vibrations and positional information to nerve impulses that travel to the brain.
- Codominance
Means that neither allele can mask the expression of the other allele.
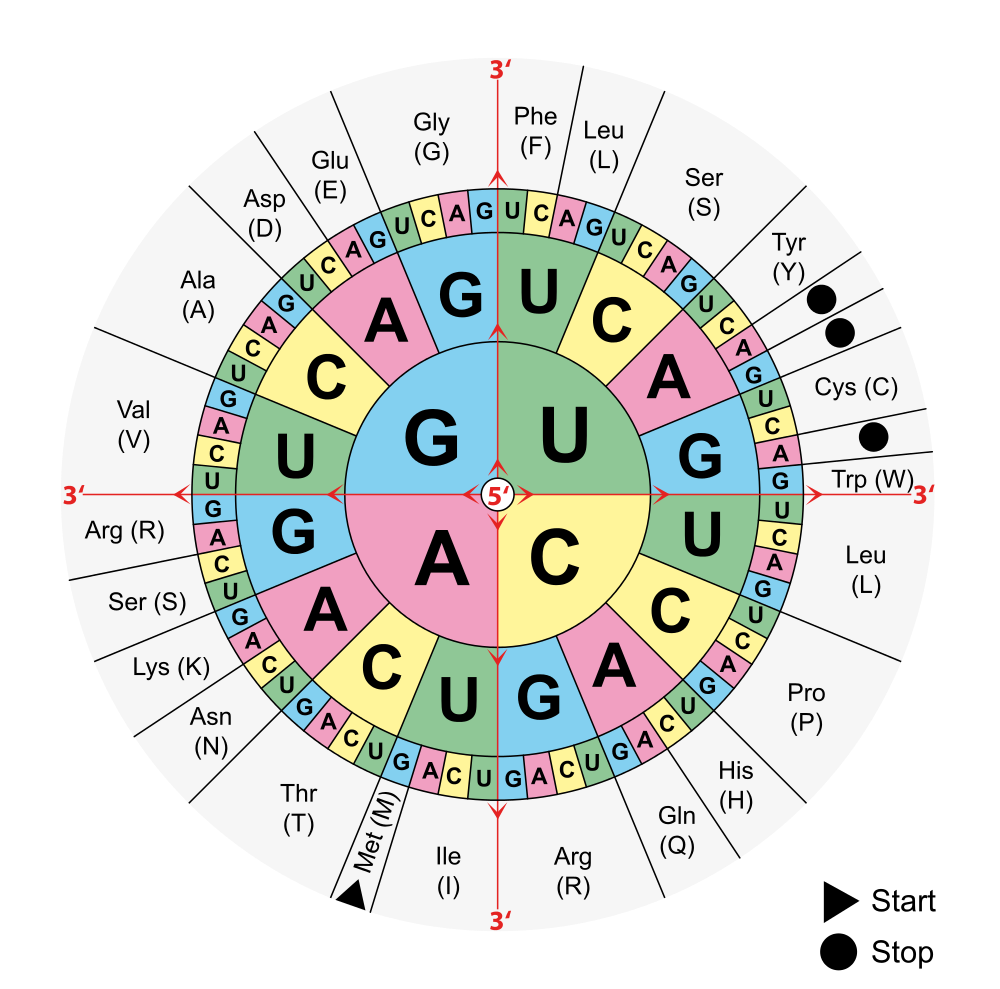
- Codon
A sequence of 3 DNA or RNA nucleotides that corresponds with a specific amino acid or stop signal during protein synthesis.
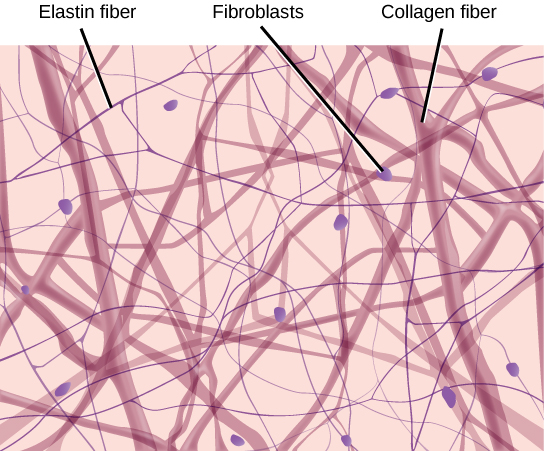
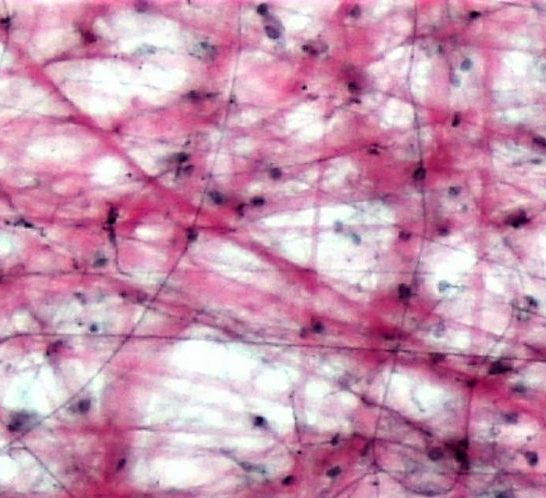
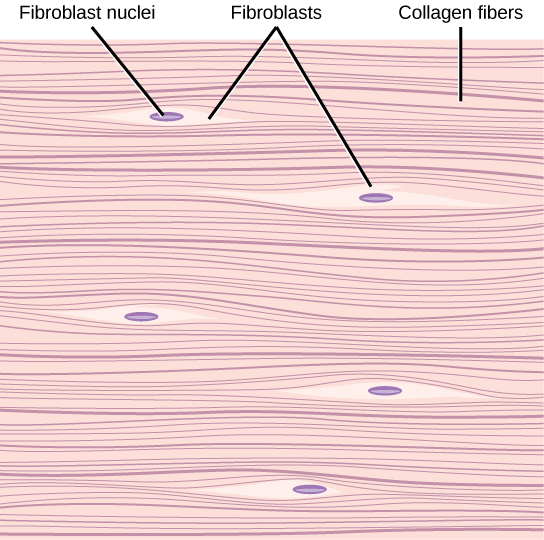
- Collagen
The main structural protein in the extracellular matrix in the various connective tissues in the body. As the main component of connective tissue, it is the most abundant protein in mammals, making up from 25% to 35% of the whole-body protein content.
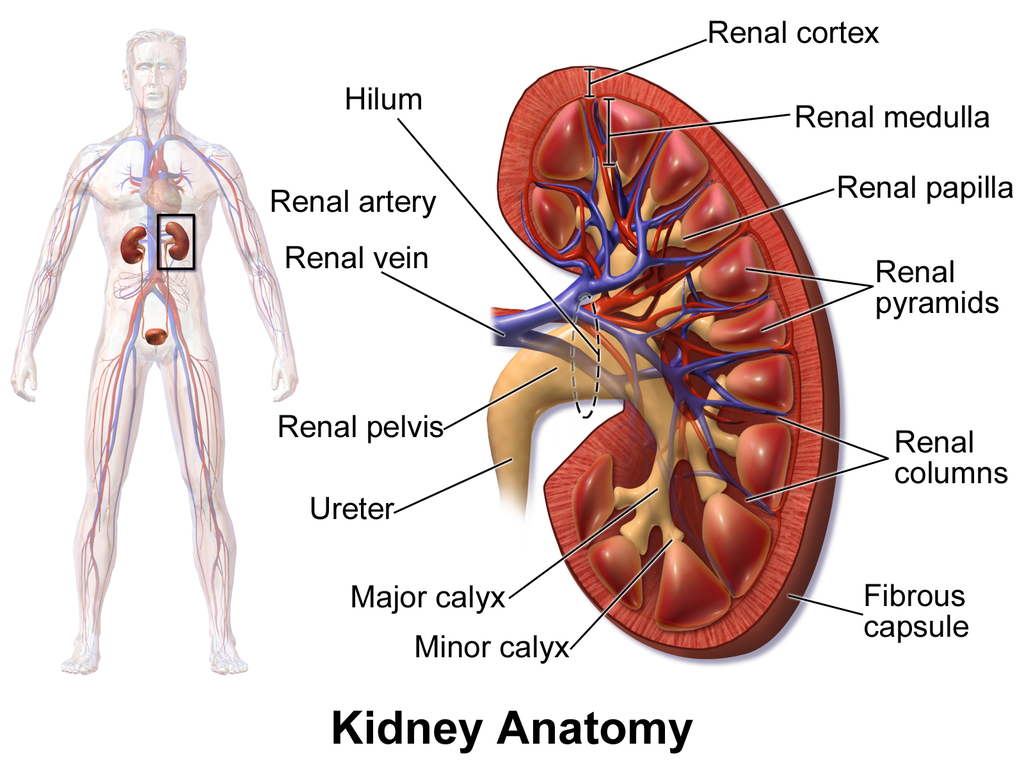
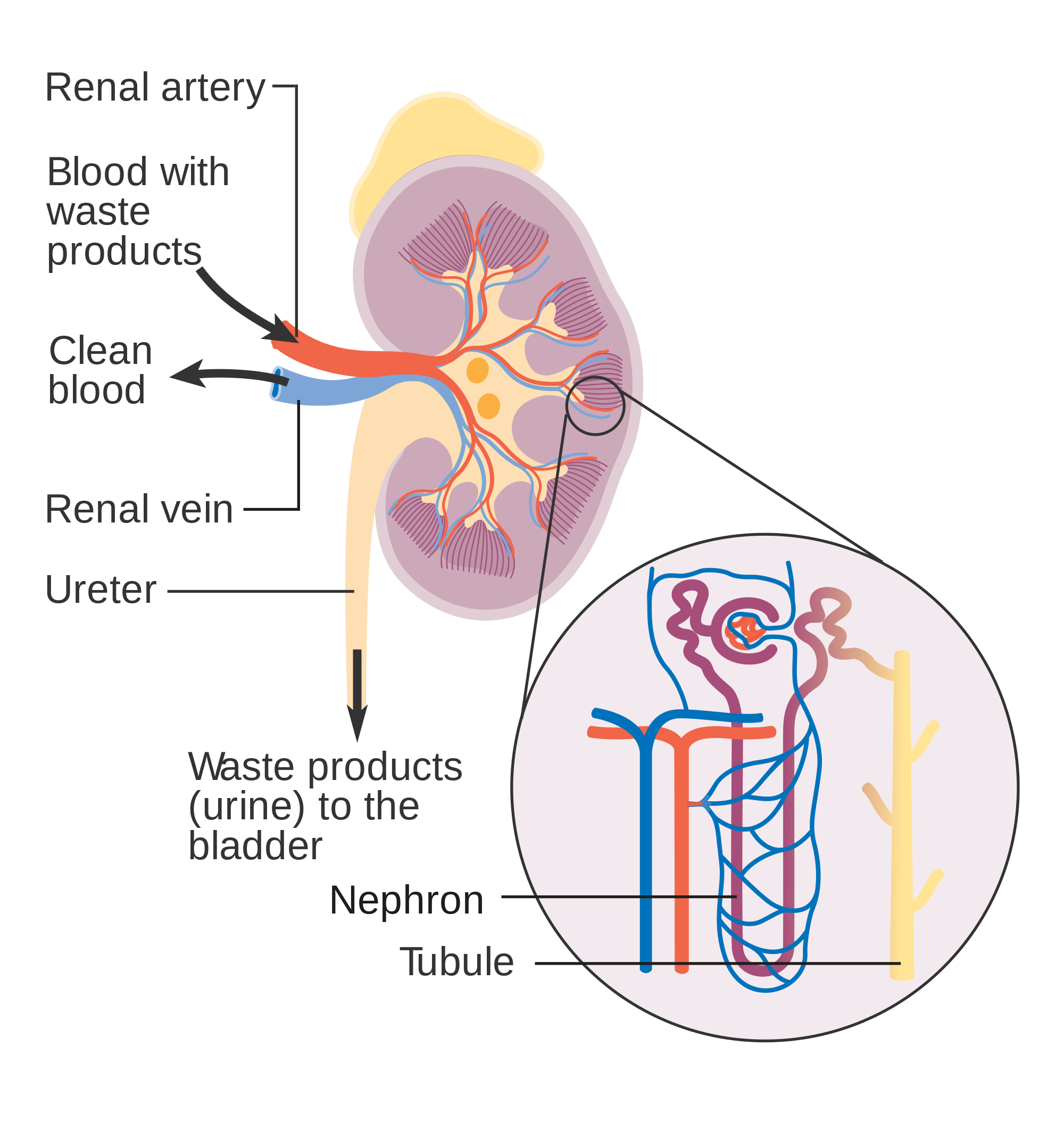
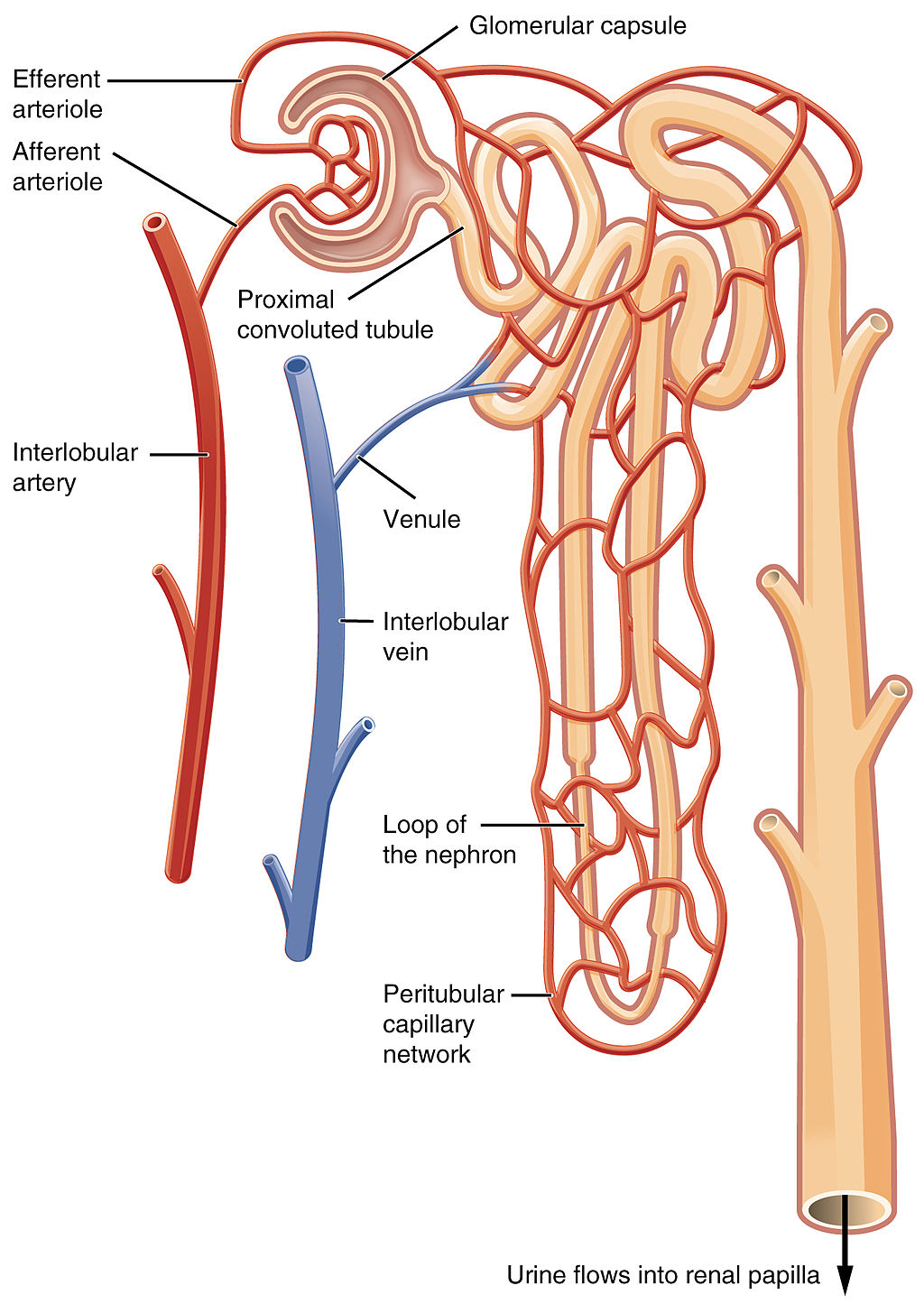
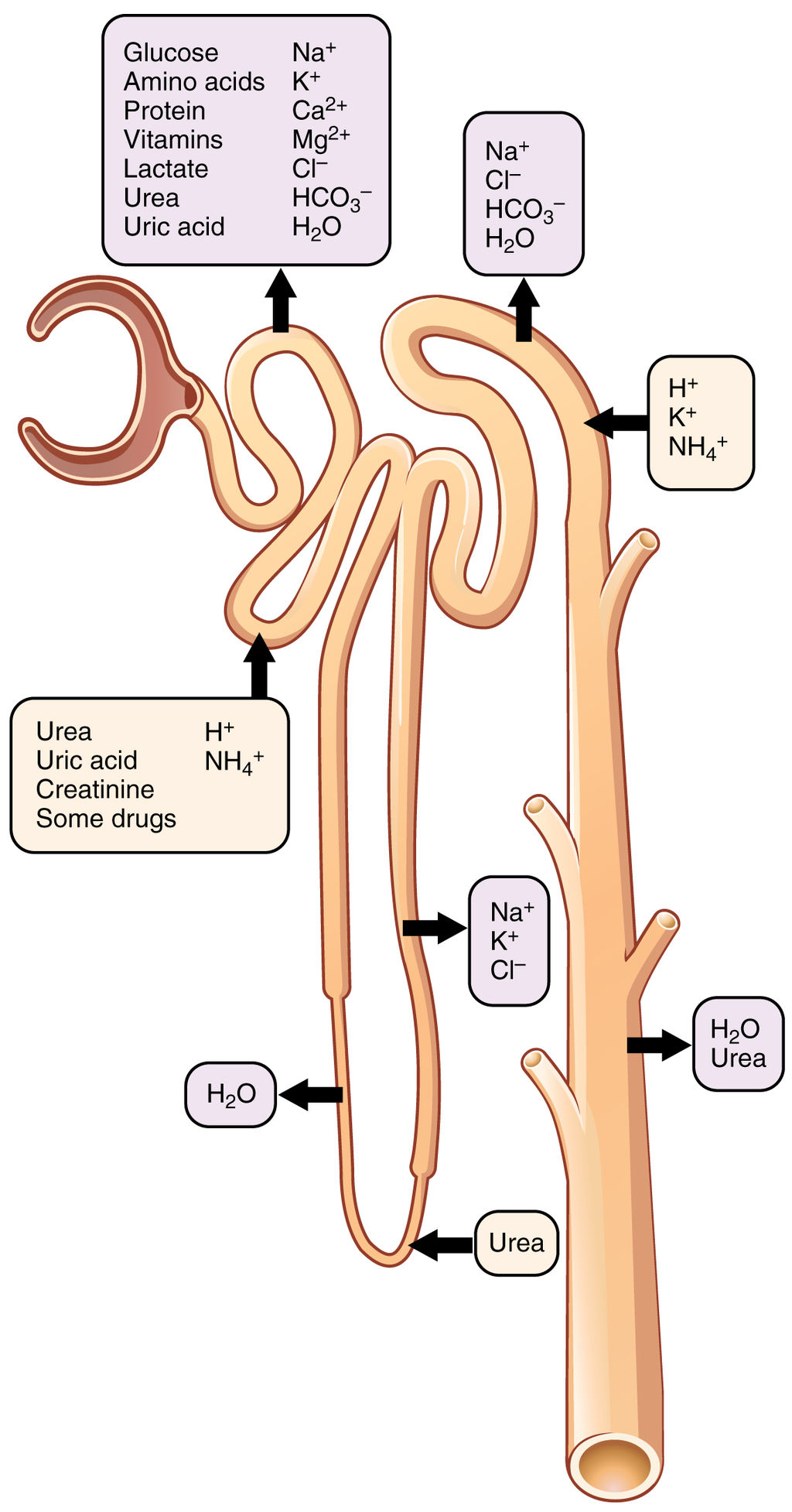
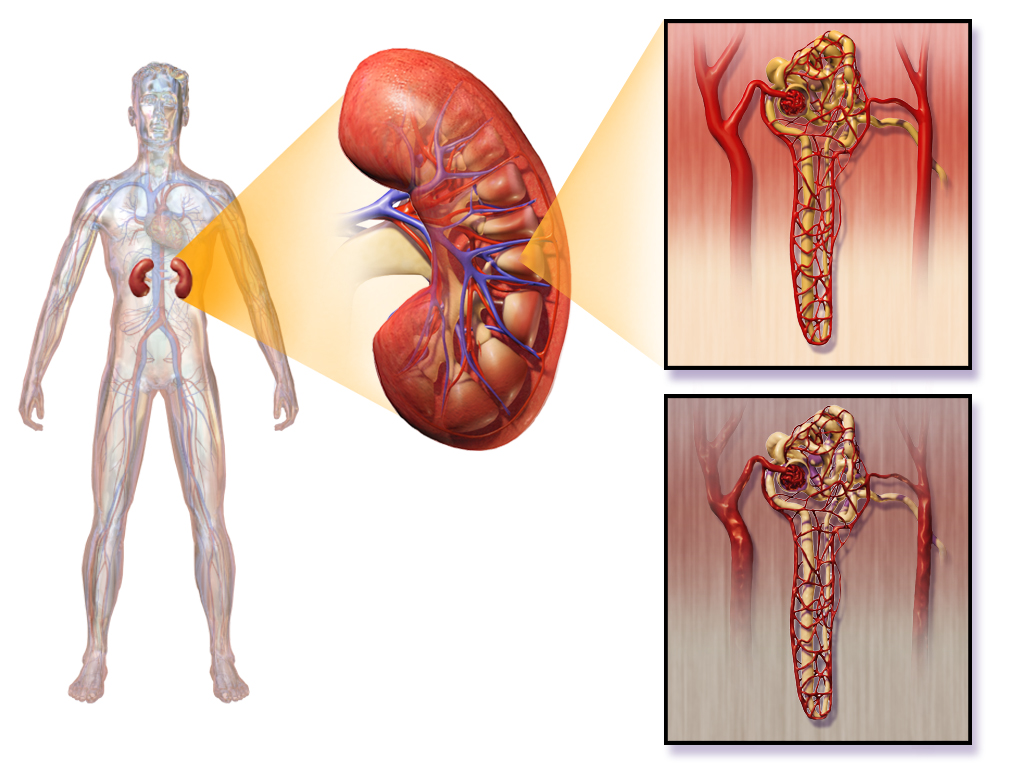
- Collecting duct
One of a network of ducts in a kidney where additional water may be reabsorbed from urine.
- Colon
The main part of the large intestine between the small intestine and rectum where water and salts are removed from liquid food wastes to form feces.
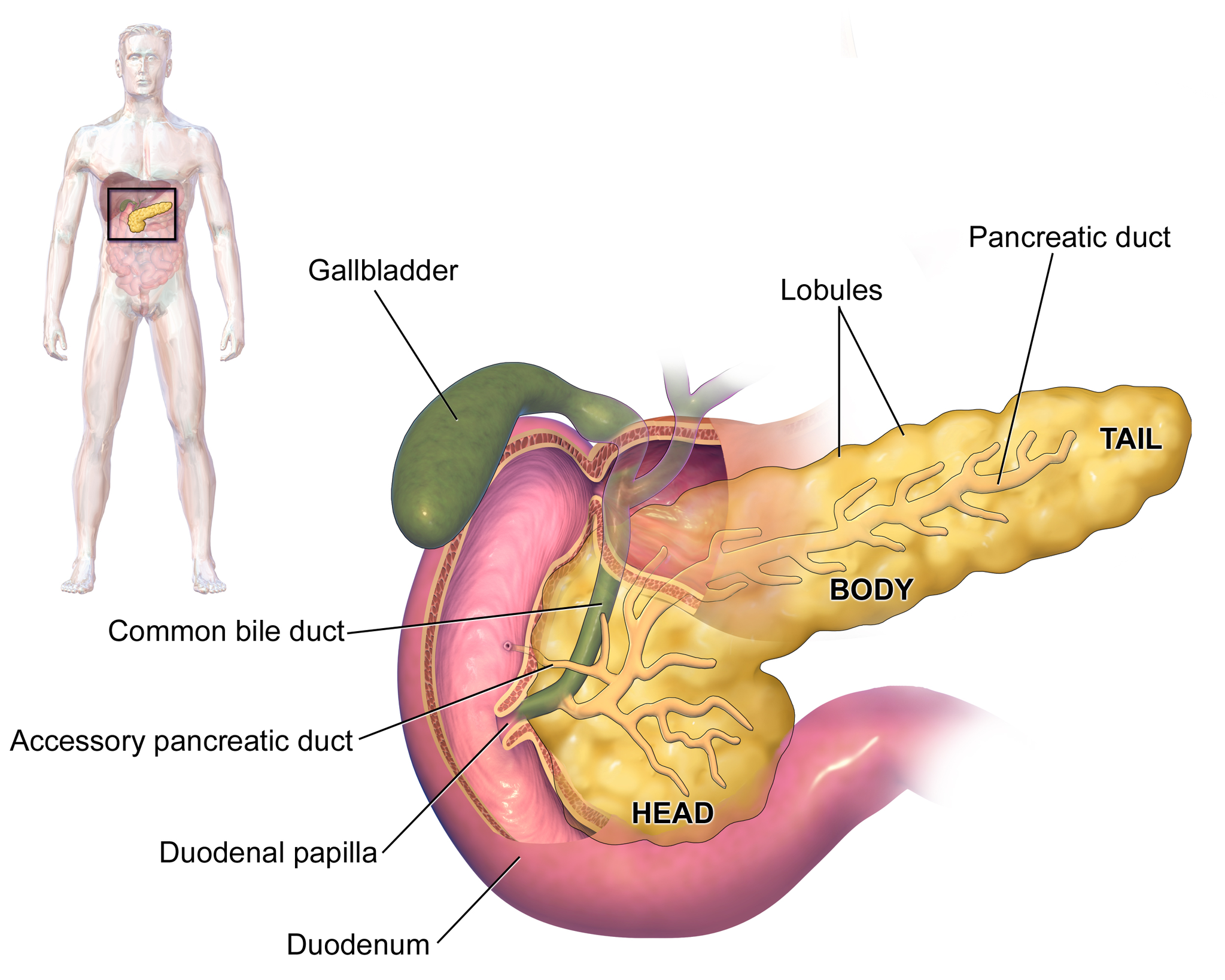
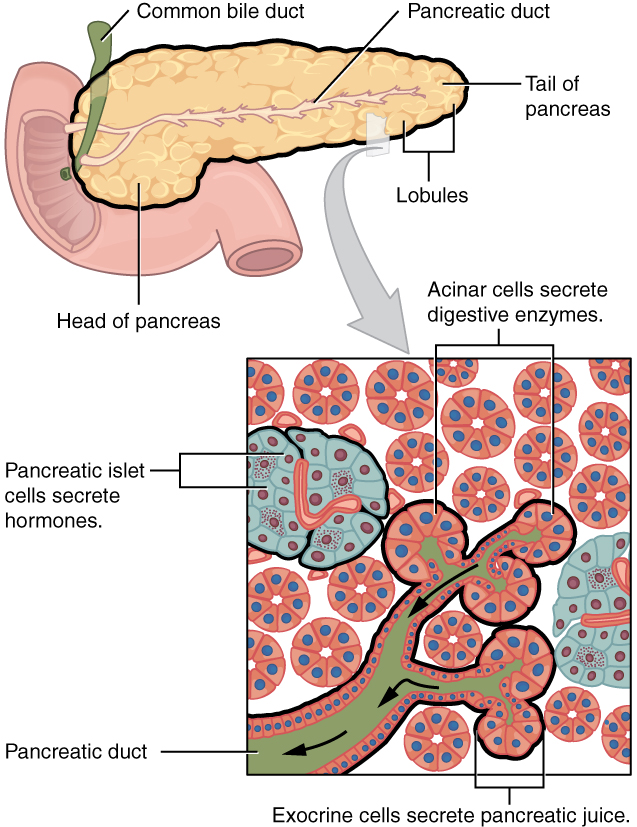
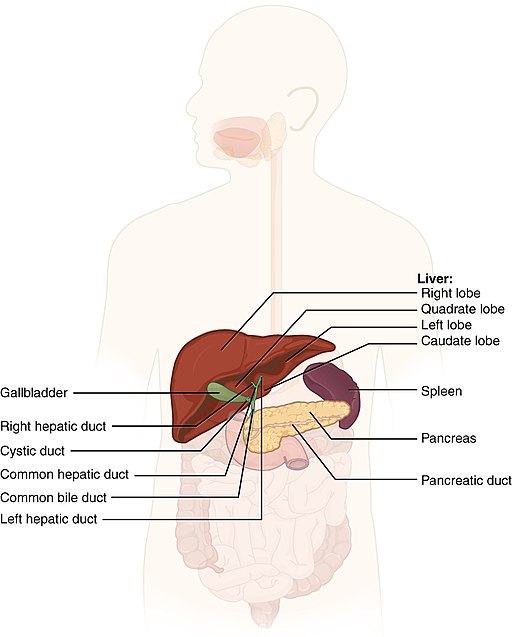
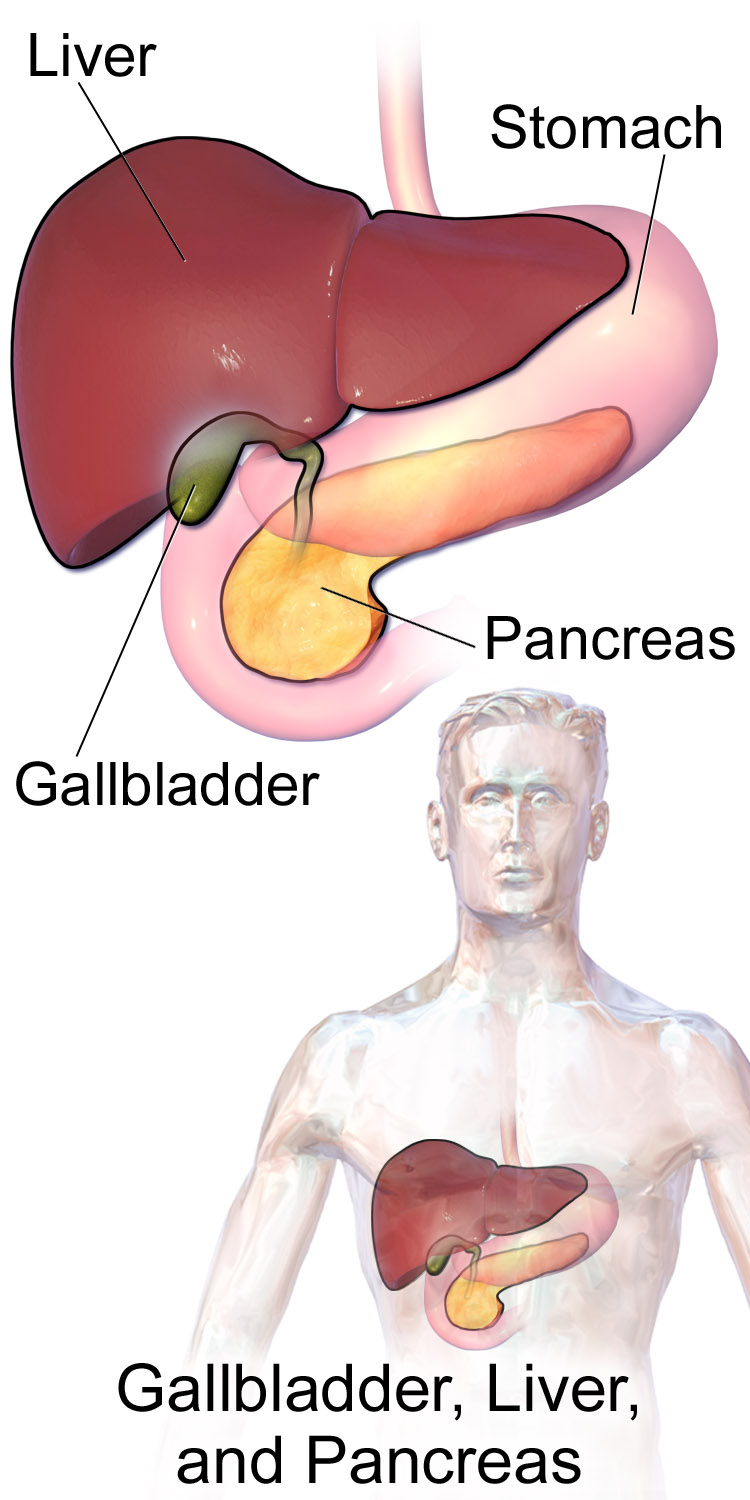
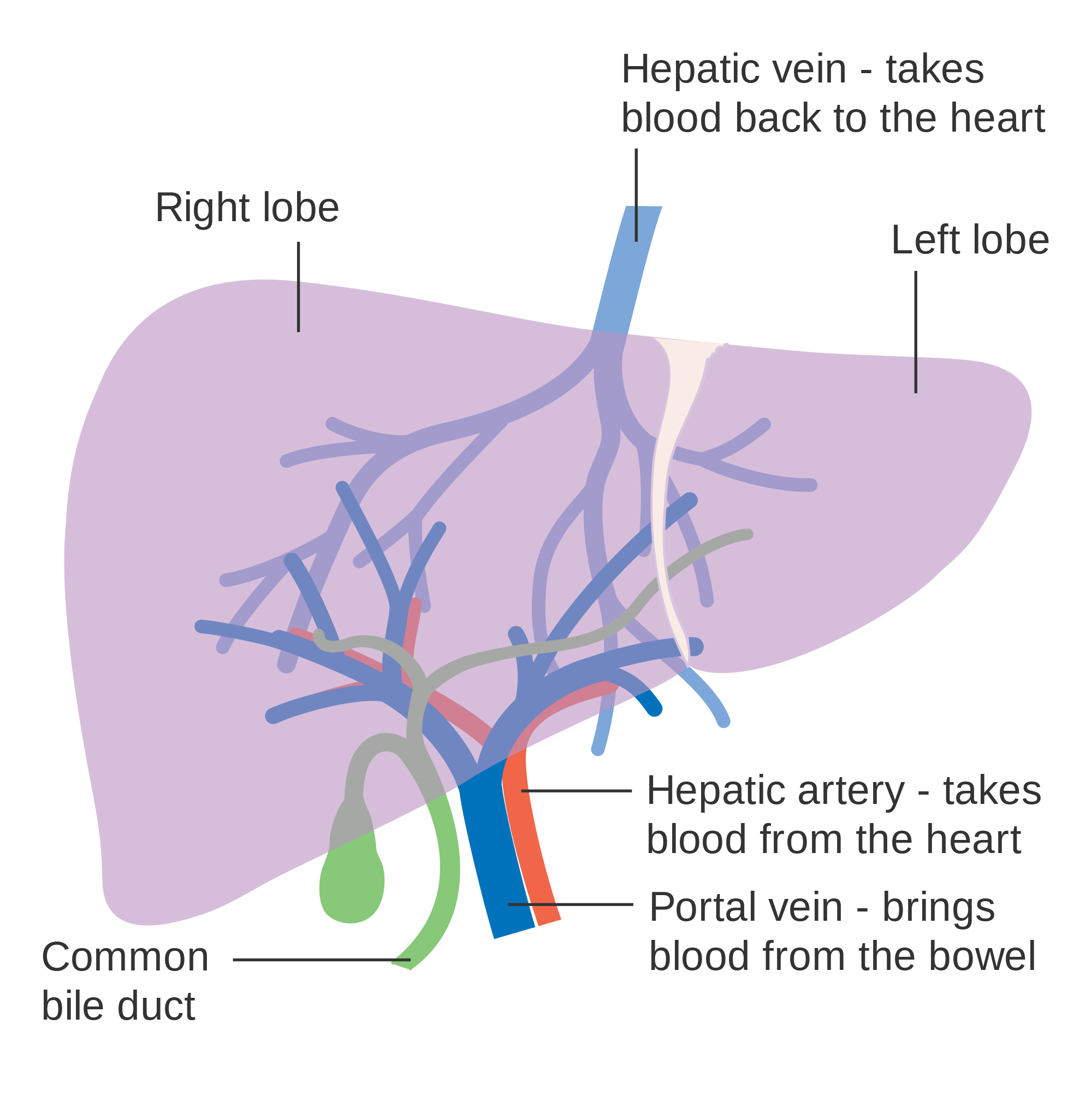
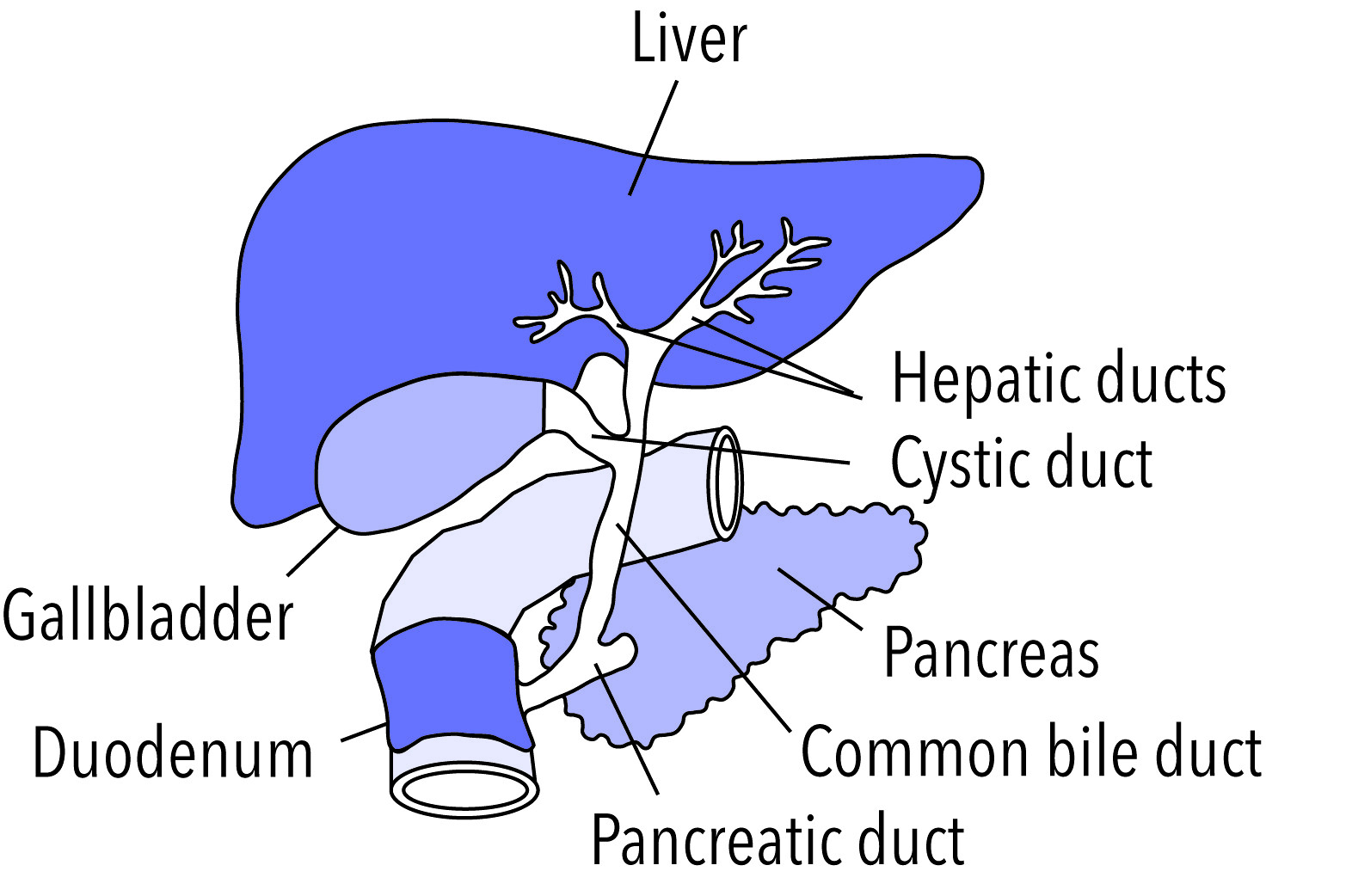
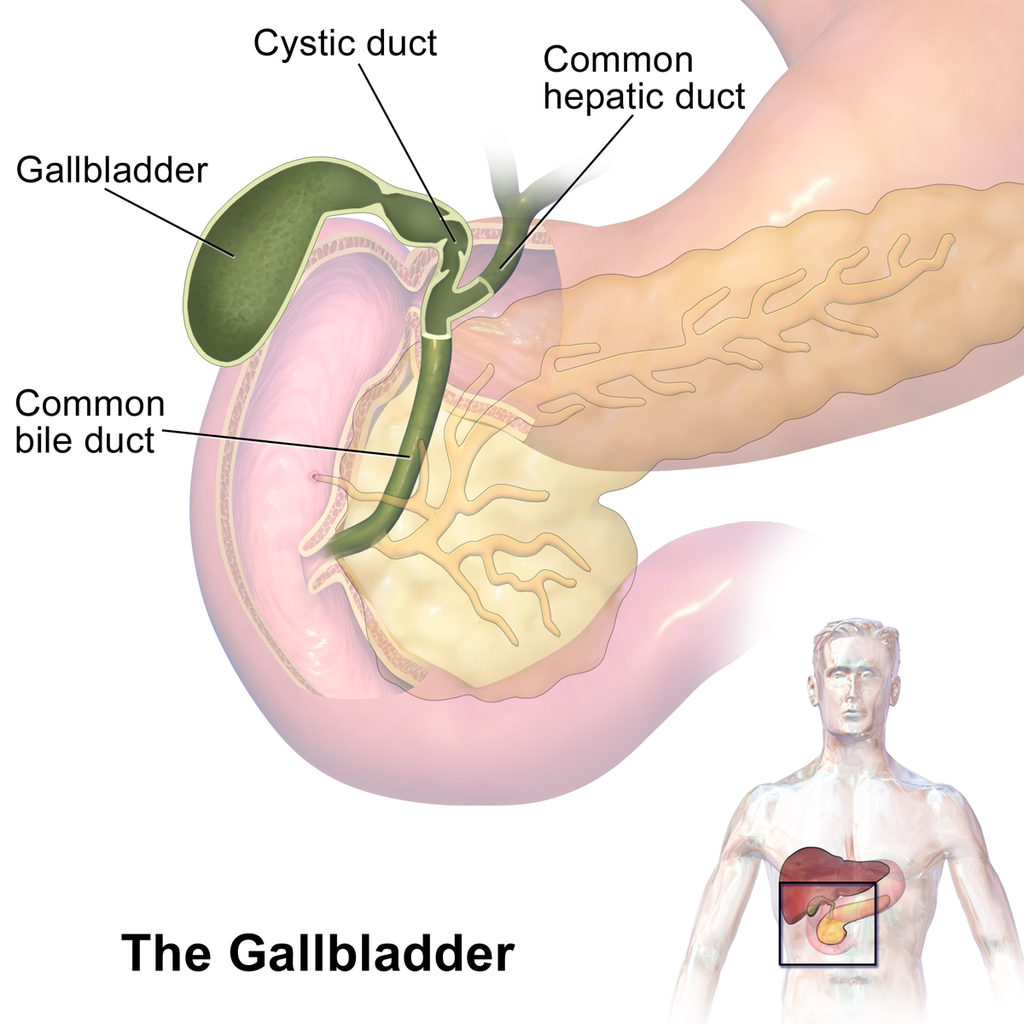
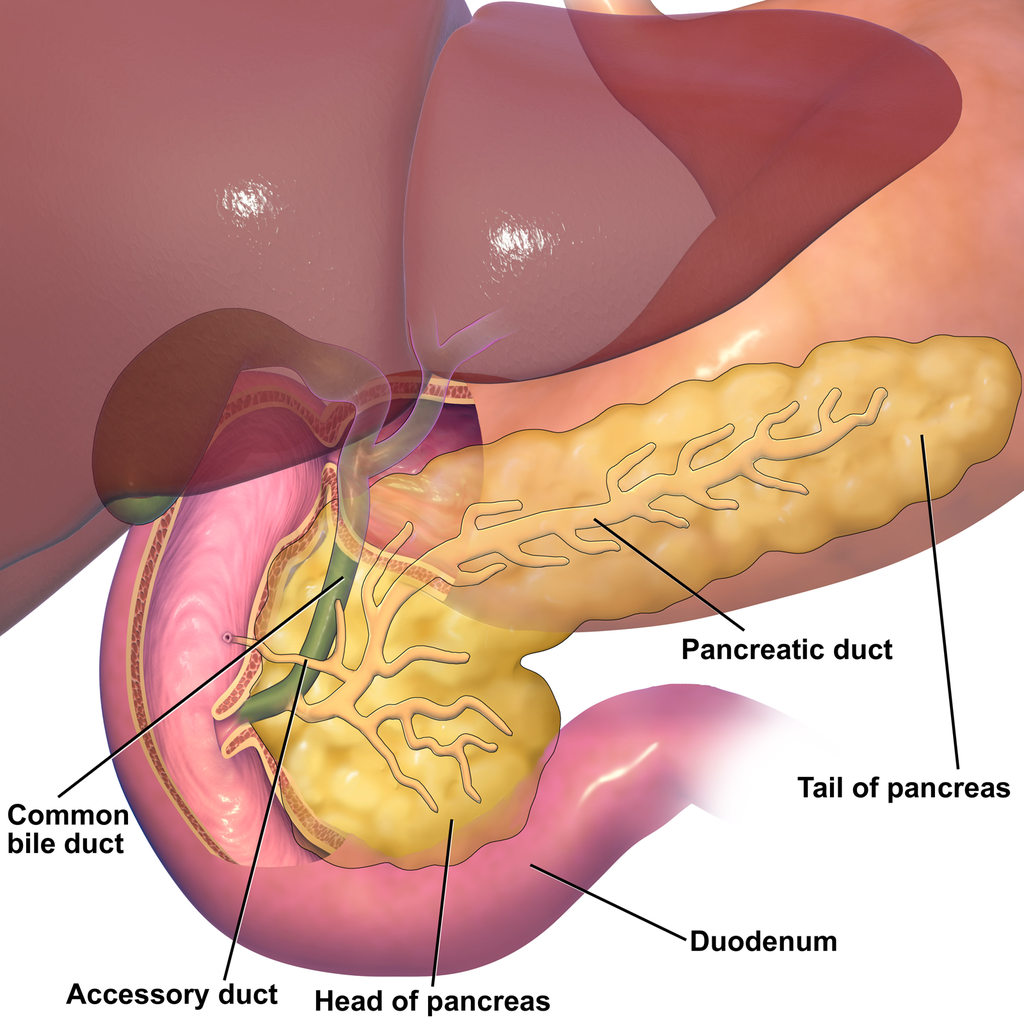
- Common bile duct
A tube that carries bile from the liver and the gallbladder through the pancreas and into the duodenum (the upper part of the small intestine). It is formed where the ducts from the liver and gallbladder are joined. It is part of the biliary duct system.
- Compact bone tissue
A type of bone tissue that is smooth and dense and makes up the outer layer of bones.
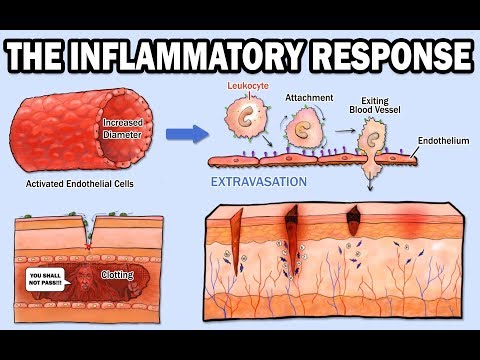
- Complement system
An innate immune response that consists of a cascade of proteins that complement the killing of pathogens by antibodies.
- Complementary base pairing
Complementary base pairing is the phenomenon where in DNA, guanine always hydrogen bonds to cytosine, and adenine always binds to thymine. In RNA, guanine always hydrogen bonds with cytosine, and adenine always hydrogen bonds with uracil.
- Complex carbohydrate
A polysaccharide (such as starch, cellulose or chitin) consisting of usually hundreds or thousands of monosaccharide units.
- Compound
A substance consisting of atoms or ions of two or more different elements in definite proportions joined by chemical bonds into a molecule.
- Concentration
The amount of particles of a substance in a given amount of solution.
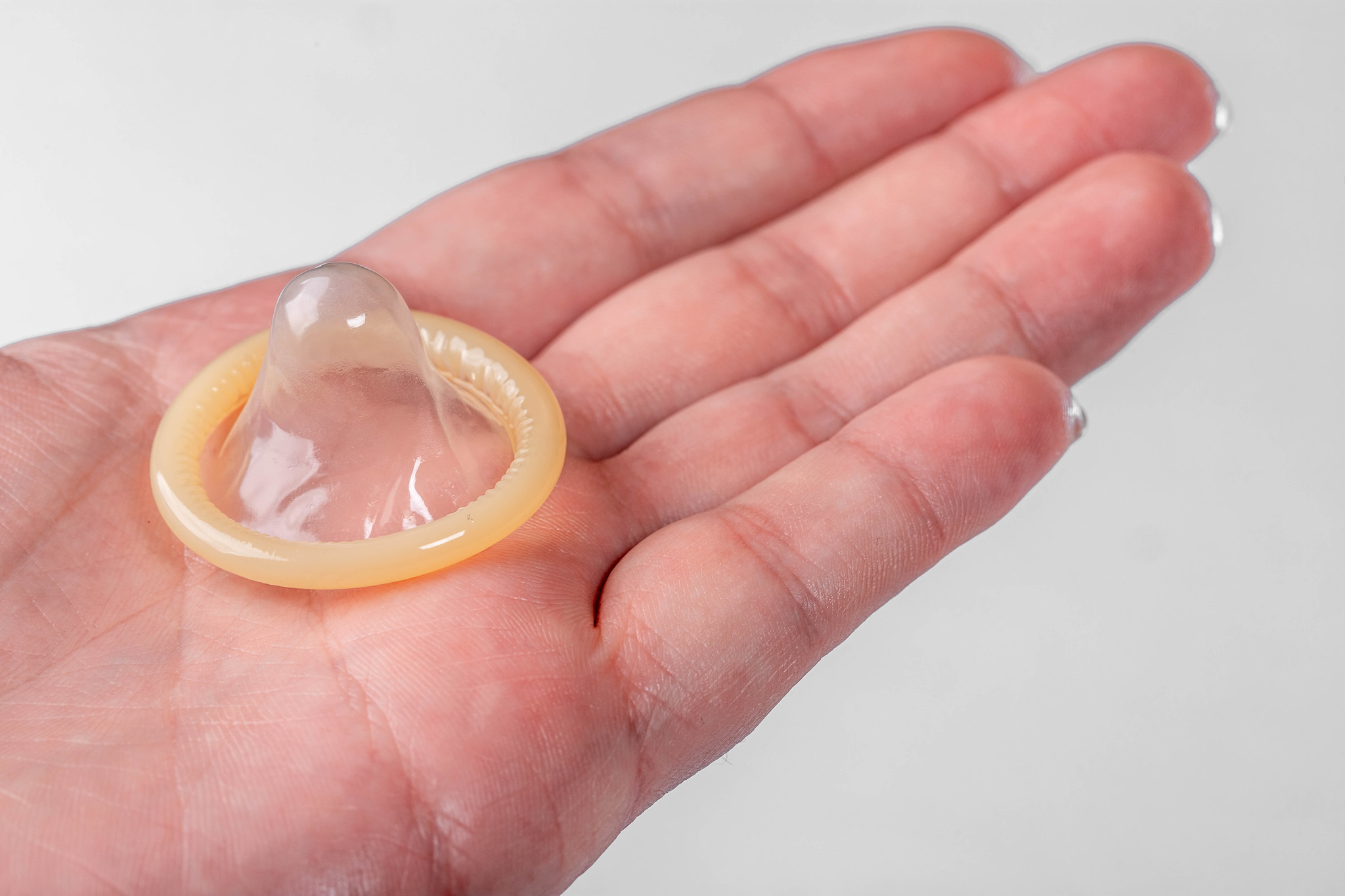
- Condom
A thin rubber sheath worn on a man's penis during sexual intercourse as a contraceptive or as a protection against infection.
- Condyloid joint
A synovial joint in which an oval-shaped process of one bone fits into a roughly elliptical cavity of the other, allowing movement in two planes.
- Cone cells
One of the two types of photoreceptor cells that are in the retina of the eye which are responsible for color vision as well as eye color sensitivity; they function best in relatively bright light, as opposed to rod cells that work better in dim light.
- Congenital disorder
A medical condition that is present at or before birth. These conditions, also referred to as birth defects, can be acquired during the fetal stage of development or from the genetic make up of the parents.
- Connective tissue
One of the four basic types of tissue, connective tissue is found in between other tissues everywhere in the body, including the nervous system and generally forms a framework and support structure for body tissues and organs.
- Consumer
Organisms that eat organisms from a different population in order to satisfy their energy needs.
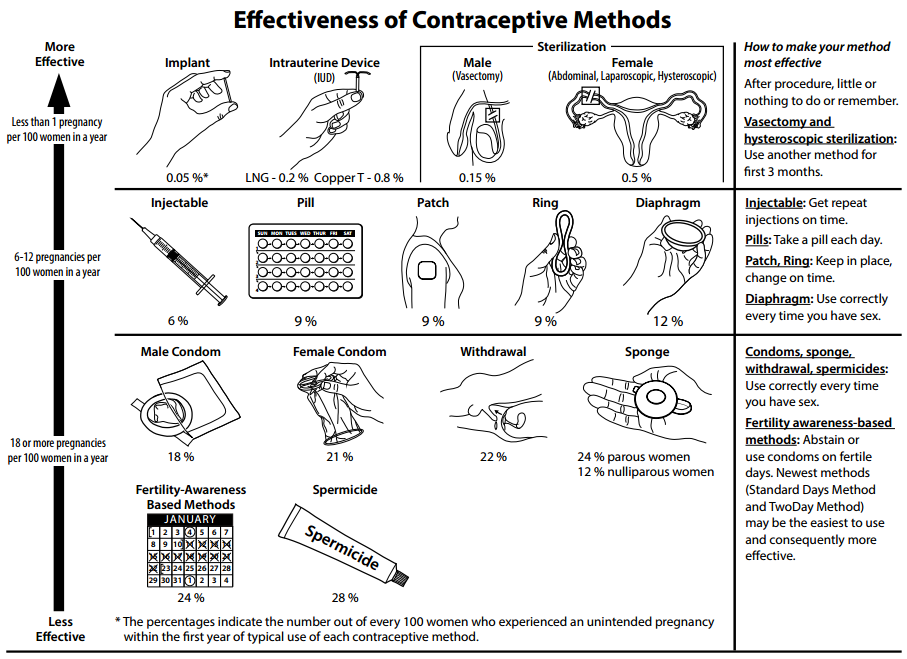
- Contraception
Any method or device used to prevent pregnancy; also called birth control.
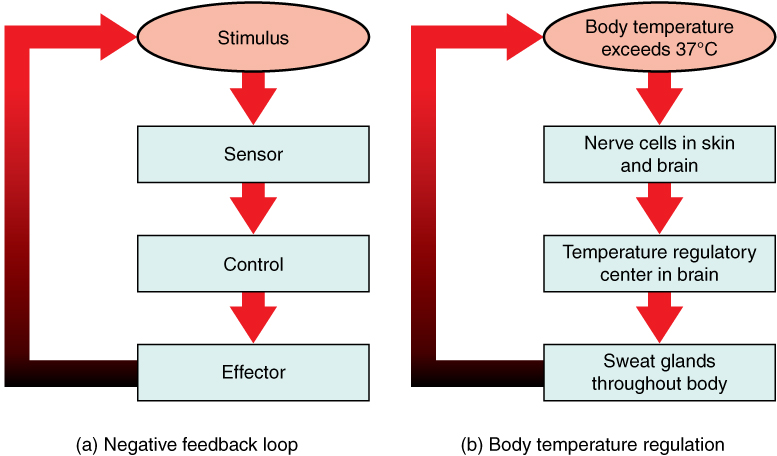
- Control center
Component of a homeostatic control mechanism that monitors a variable and sends signals to the effector as needed to keep the variable in homeostasis.
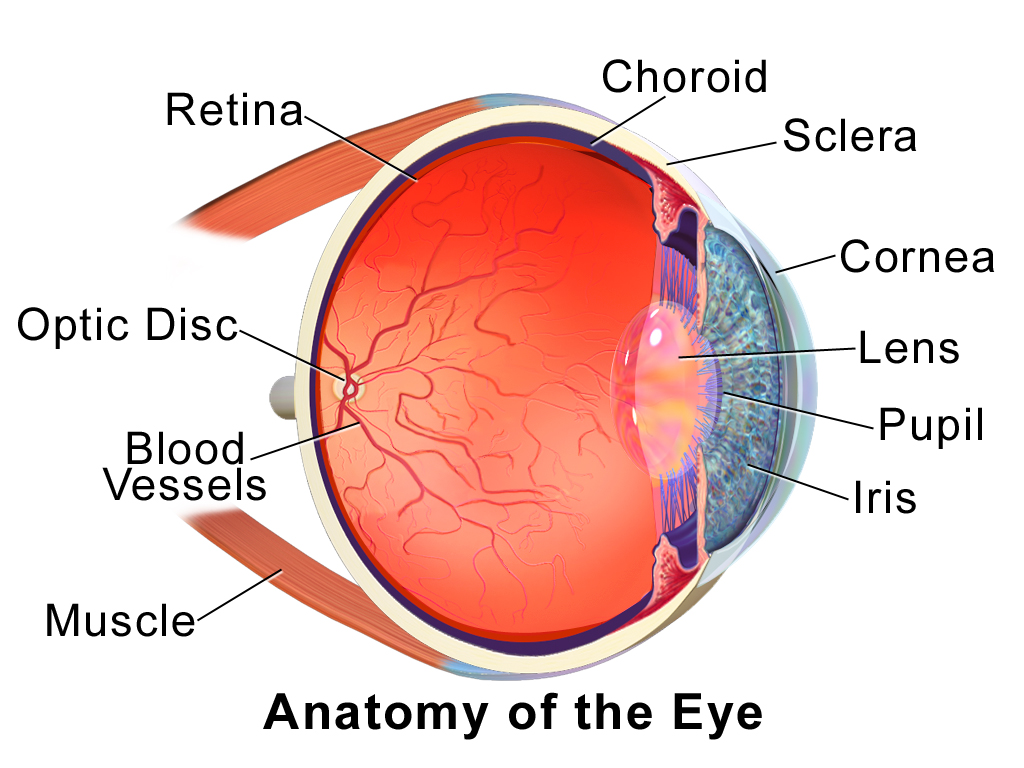
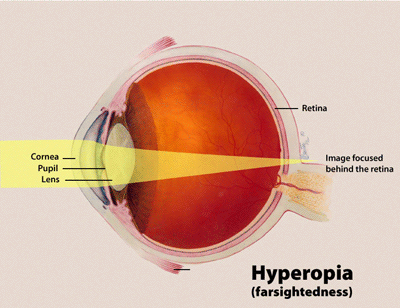
- Cornea
The transparent front part of the eye that covers the iris, pupil, and anterior chamber.
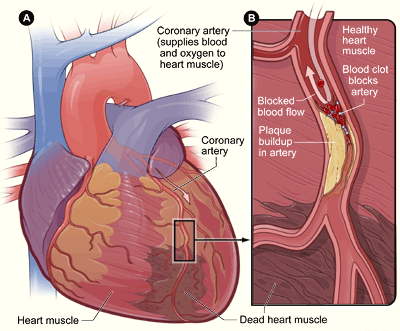
- Coronary artery
One of two arteries that supply the cells of the heart with oxygen and nutrients.
- Coronary artery disease
A class of diseases that result from atherosclerosis of coronary arteries; includes angina and myocardial infarction (heart attack).
- Coronary circulation
Part of the systemic circulatory system that supplies blood to and provides drainage from the tissues of the heart.
- Corpus callosum
A thick band of nerve fibers that divides the cerebral cortex lobes into left and right hemispheres. It connects the left and right sides of the brain, allowing for communication between both hemispheres.
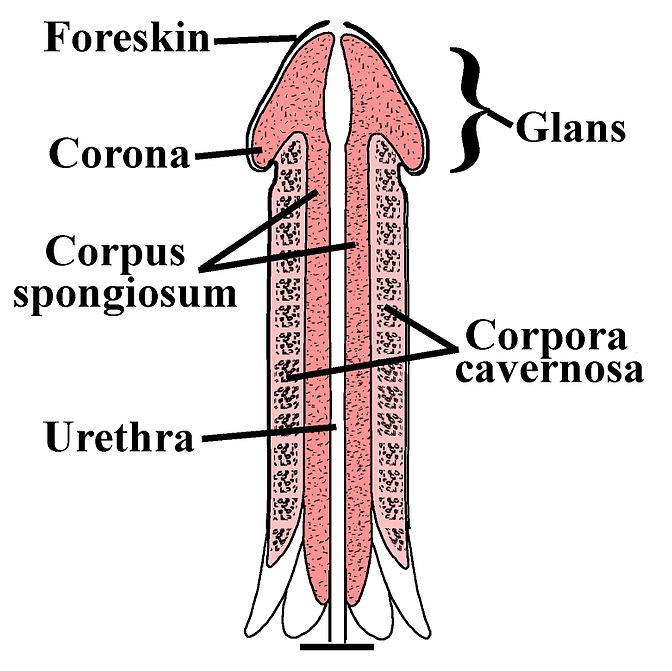
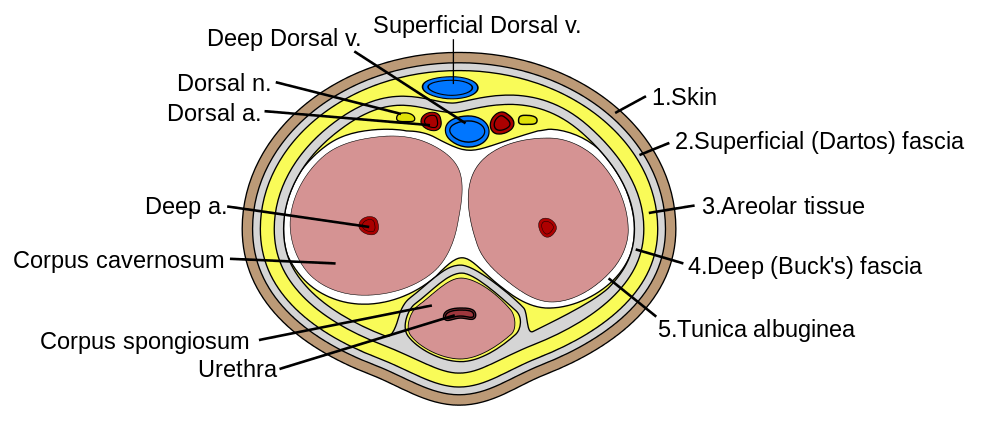
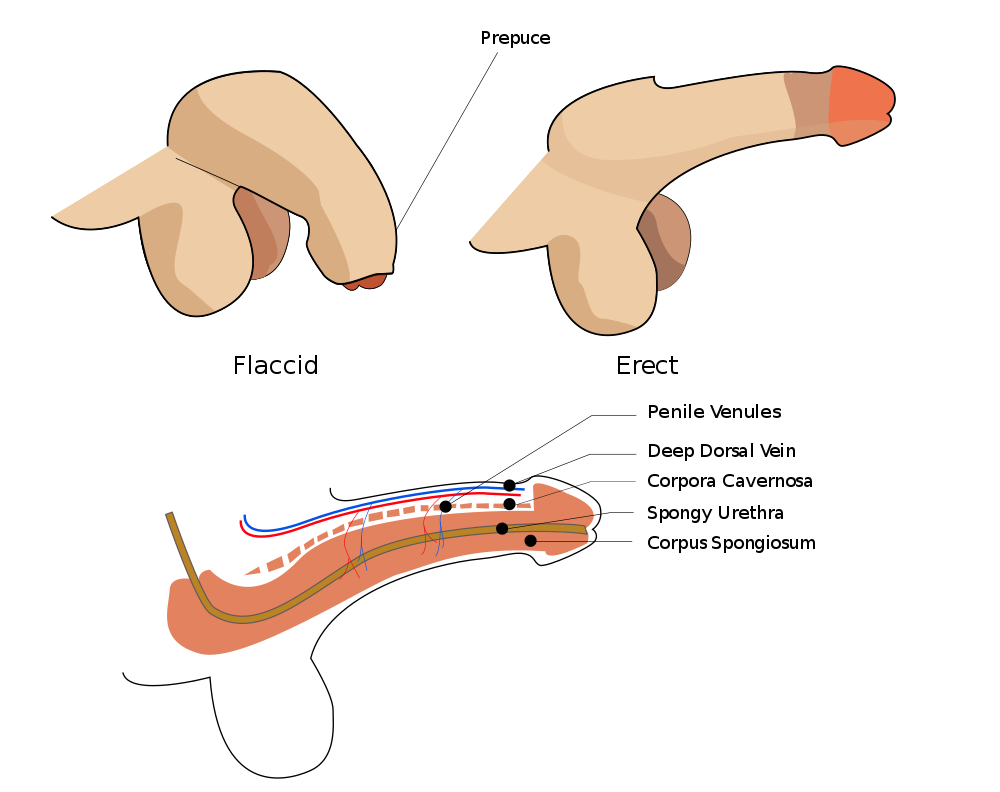
- Corpus cavernosum
Either of two masses of erectile tissue forming the bulk of the penis and the clitoris.
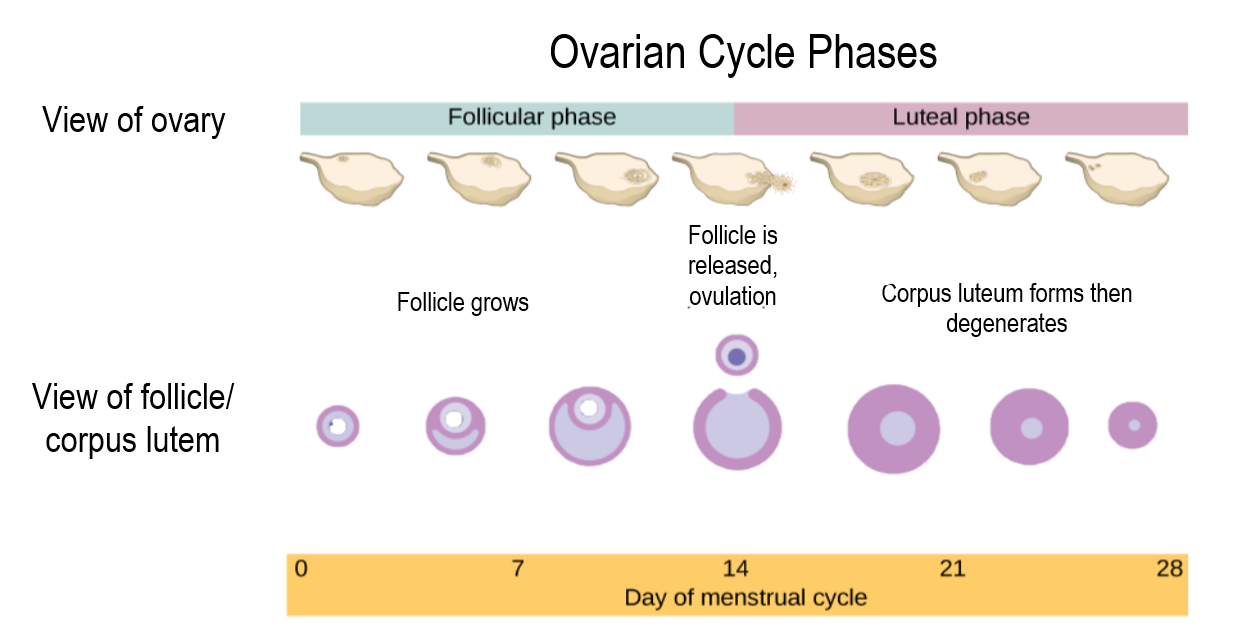
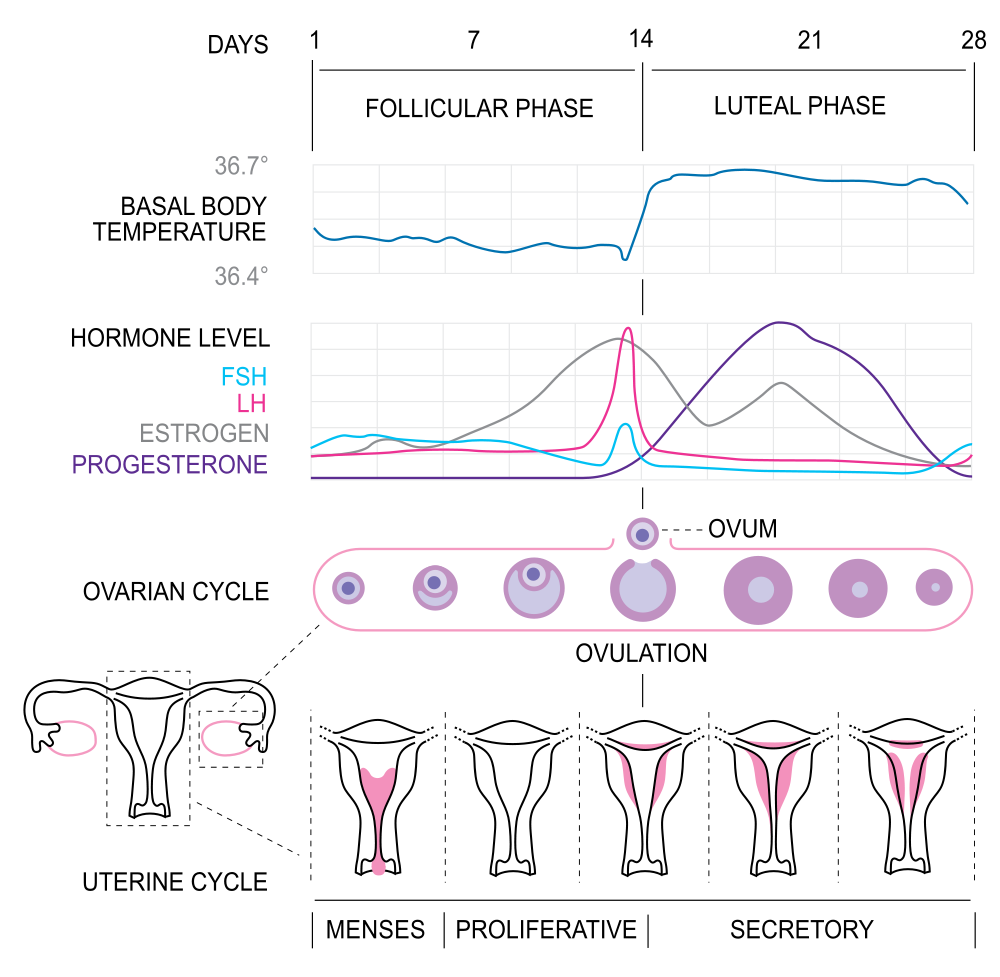
- Corpus luteum
An ovarian structure that forms from a follicle after it matures and ovulates an egg.
- Corpus spongiosum
A mass of erectile tissue alongside the corpora cavernosa of the penis and terminating in the glans.
- Corticosteroid
Any steroid hormone produced by the cortex of the adrenal gland; includes mineralocorticoids, glucocorticoids, and androgens.
- Cortisol
A glucocorticoid hormone produced by the cortex of the adrenal gland that is released in response to stress and also helps control metabolic rate, suppression of the immune system, and other functions
- Cranial cavity
A cavity that fills most of the upper part of the skull and contains the brain.
- Cranium
The upper part of the skull that encloses and protects the brain.
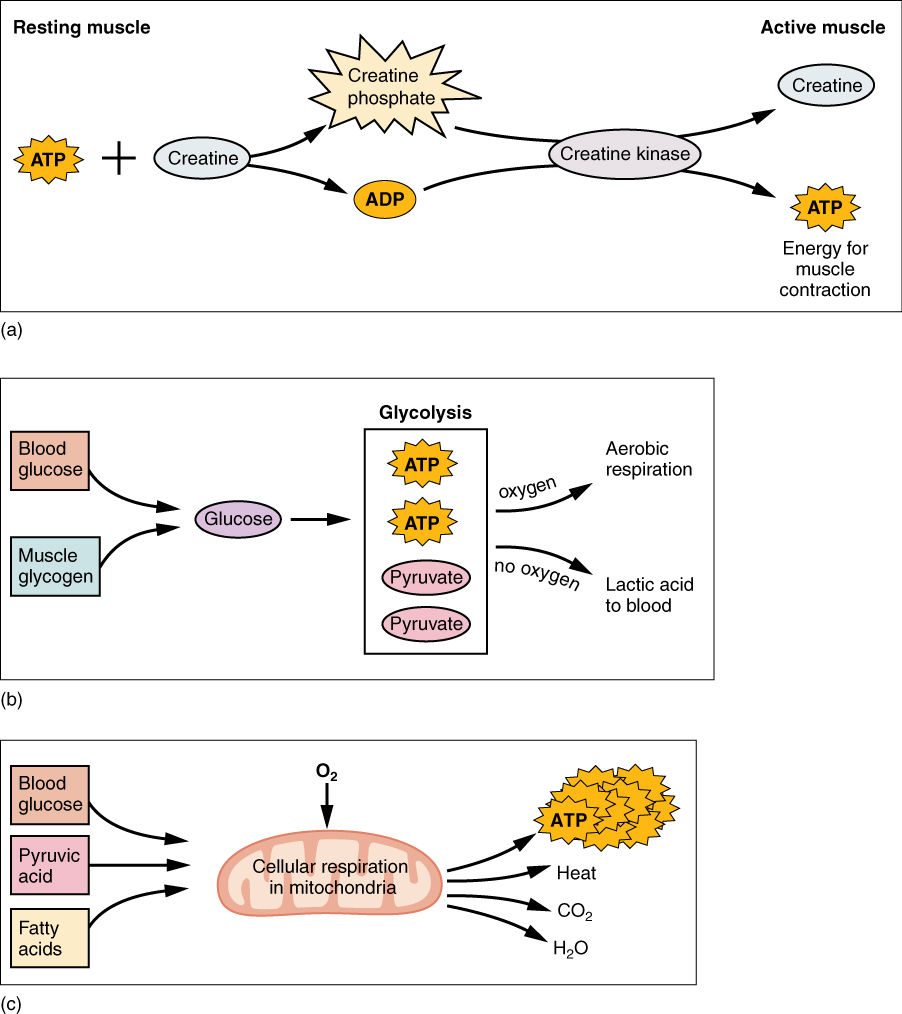
- Creatine phosphate
An organic compound of creatine and phosphate, also known as phosphocreatine, which when hydrolyzed (split apart) releases energy for muscle contraction.
- Crohn’s disease
An inflammatory bowel disease that may affect any part of the gastrointestinal tract from the mouth to the anus.
- Cross-pollination
When one plant pollinates a plant of another variety. The two plants' genetic material combines and the resulting seeds from that pollination will have characteristics of both varieties and is a new variety.
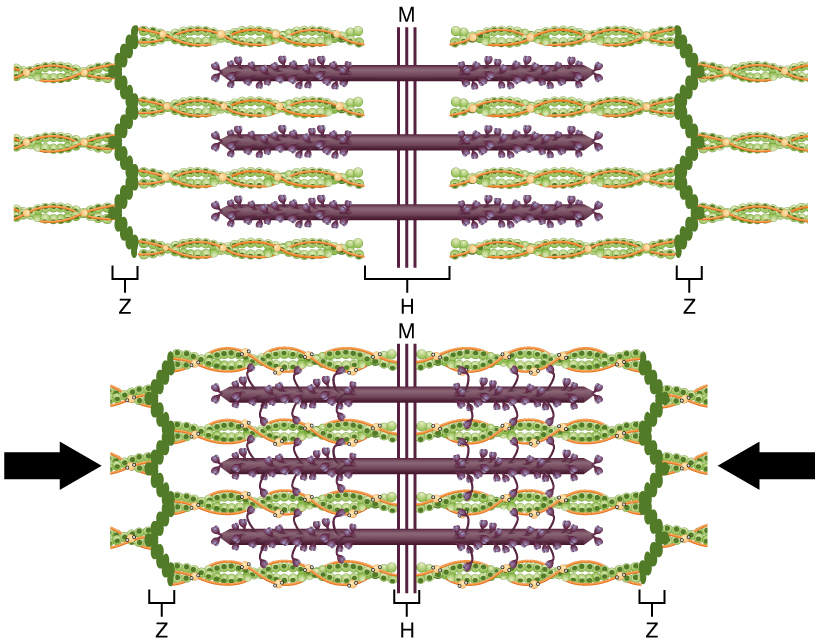
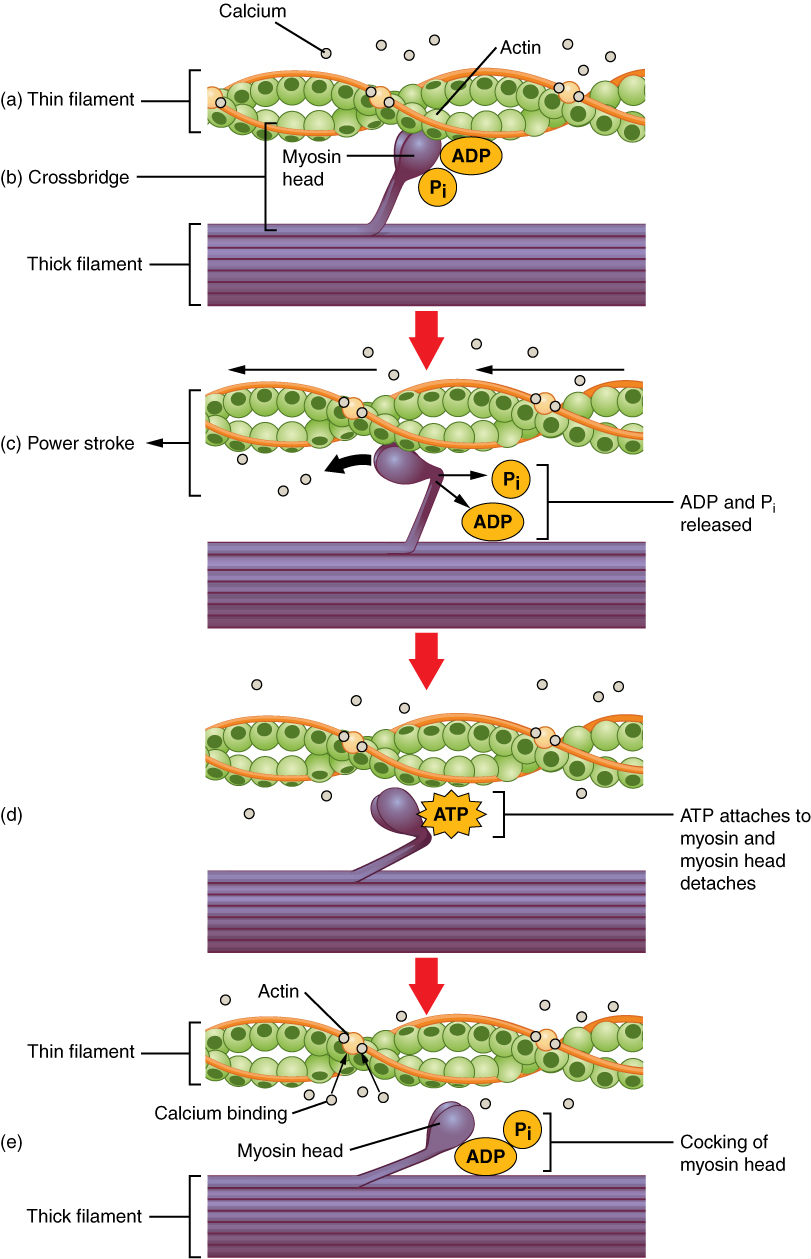
- Crossbridge cycling
A sequence of molecular events that forms crossbridges between myosin and actin filaments in muscle fibers, allowing for muscle contraction. "Heads" on the myosin filaments essentially form a connection with specific locations on the actin, and then the head bends in order to pull the myosin strand along the actin to shorten the sarcomere.
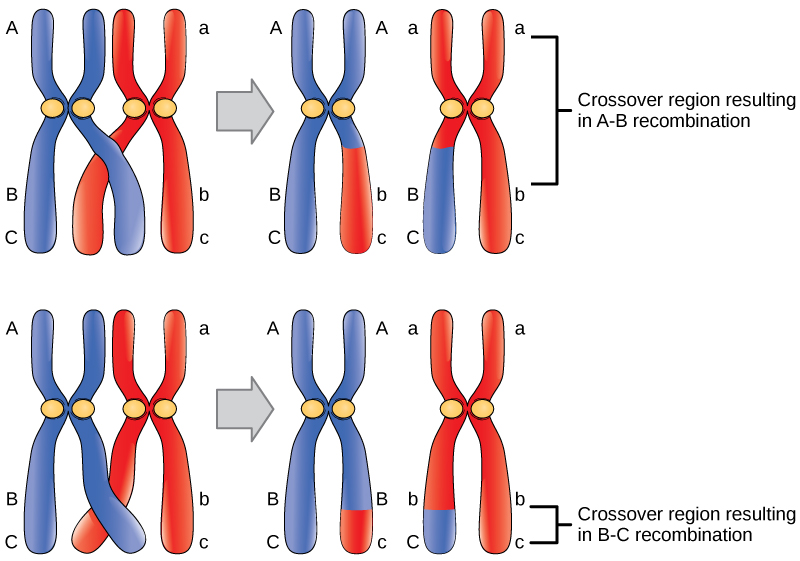
- Crossing-over
The exchange of genetic material between two homologous chromosomes non-sister chromatids that results in recombinant chromosomes during sexual reproduction.
- Cushing’s syndrome
A disorder in which there is hypersecretion of the adrenal cortex hormone cortisol, most commonly due to a tumor of the pituitary gland.
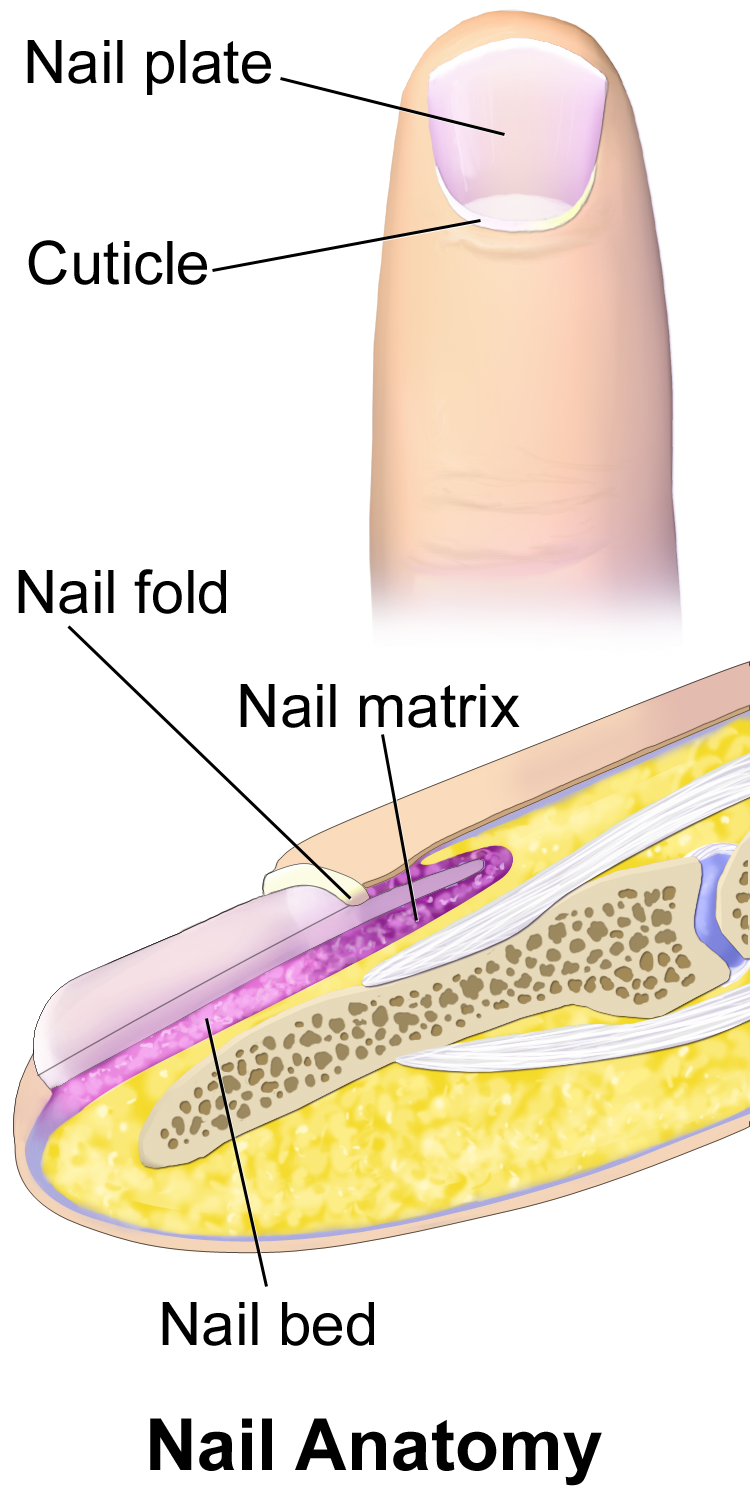
- Cuticle
A layer of clear skin located along the bottom edge of your finger or toe. The cuticle function is to protect new nails from bacteria when they grow out from the nail root. The area around the cuticle is delicate. It can get dry, damaged, and infected.
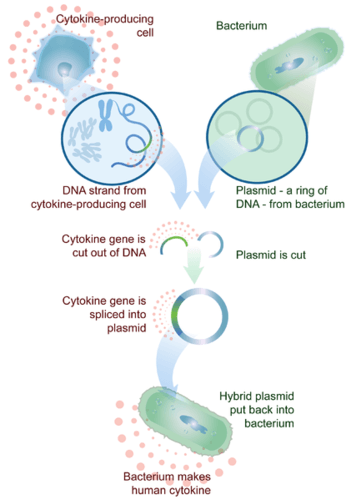
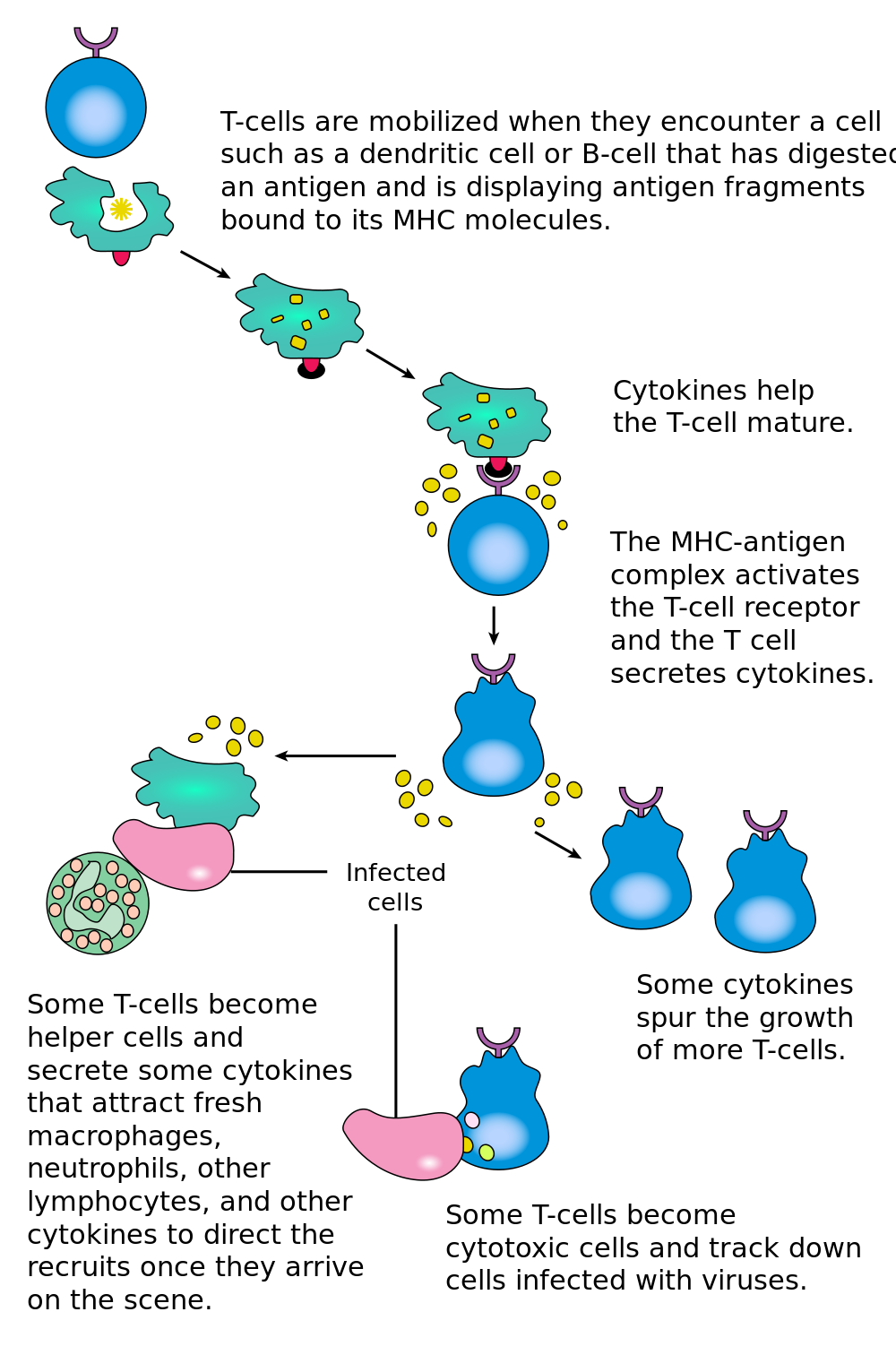
- Cytokine
A chemical released by injured, infected, or immune cells that triggers inflammation or other immune responses.
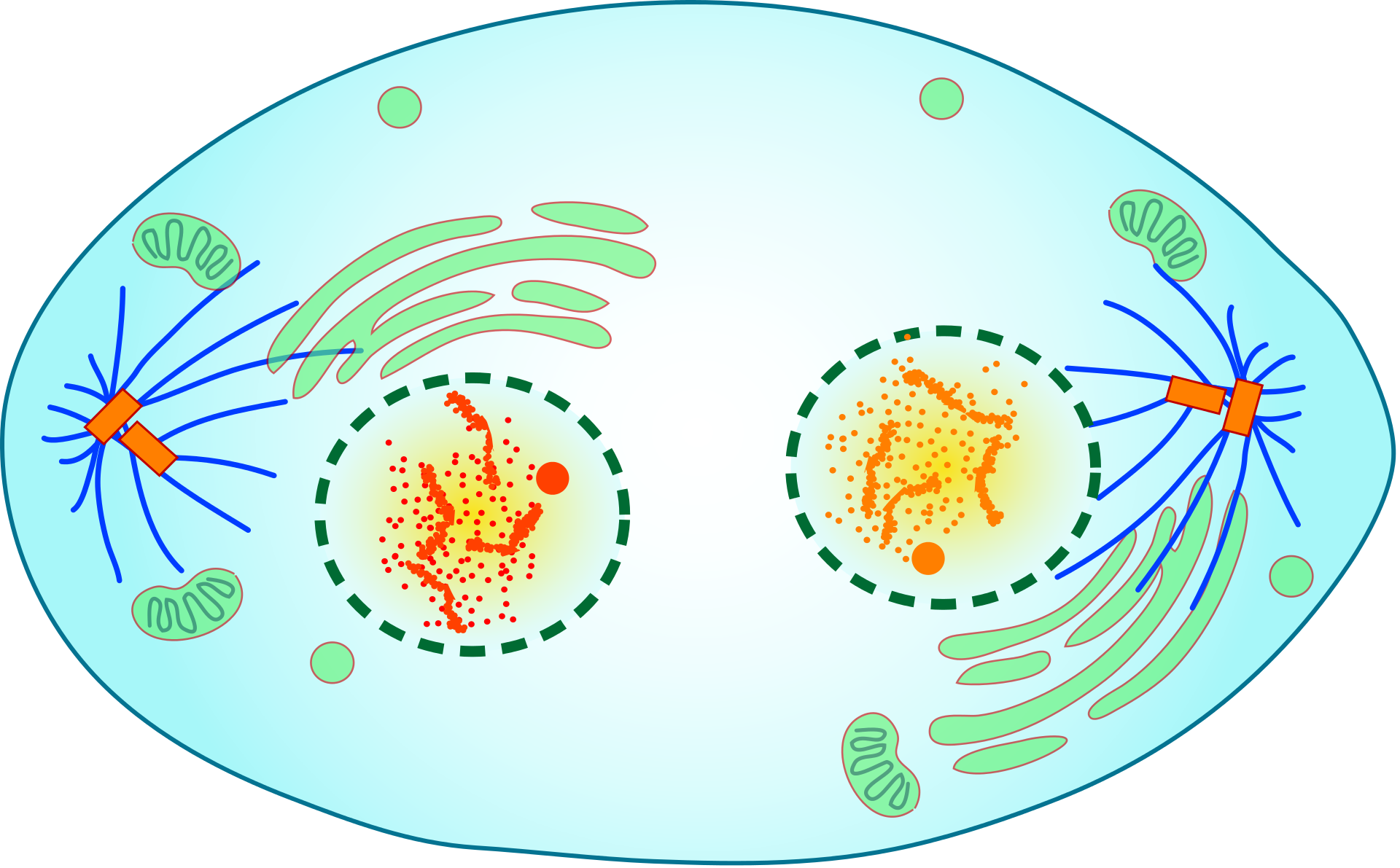
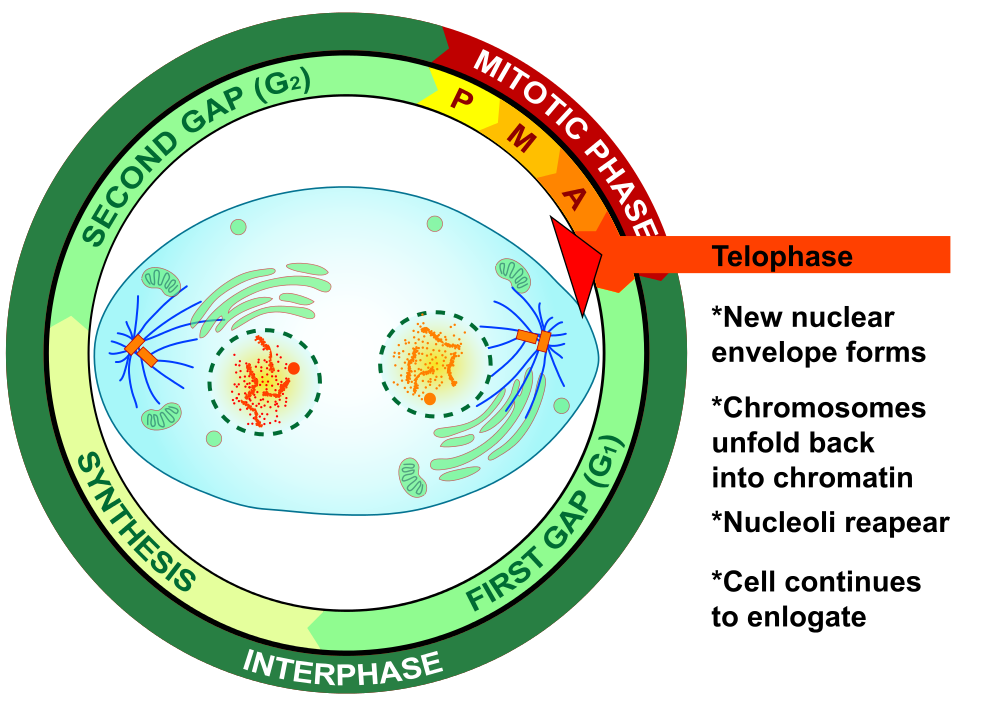
- Cytokinesis
The part of the cell division process during which the cytoplasm of a single eukaryotic cell divides into two daughter cells. Cytoplasmic division begins during or after the late stages of nuclear division in mitosis and meiosis.
- Cytoplasm
The jellylike material that makes up much of a cell inside the cell membrane, and, in eukaryotic cells, surrounds the nucleus. The organelles of eukaryotic cells, such as mitochondria, the endoplasmic reticulum, and (in green plants) chloroplasts, are contained in the cytoplasm.
- Cytoskeleton
A complex network of interlinking protein filaments that extends from the cell nucleus to the cell membrane, gives the cell its shape and help organize the cell's parts.
- Cytosol
The aqueous component of the cytoplasm of a cell, within which various organelles and particles are suspended.
- Data
Facts and statistics collected together for reference or analysis.
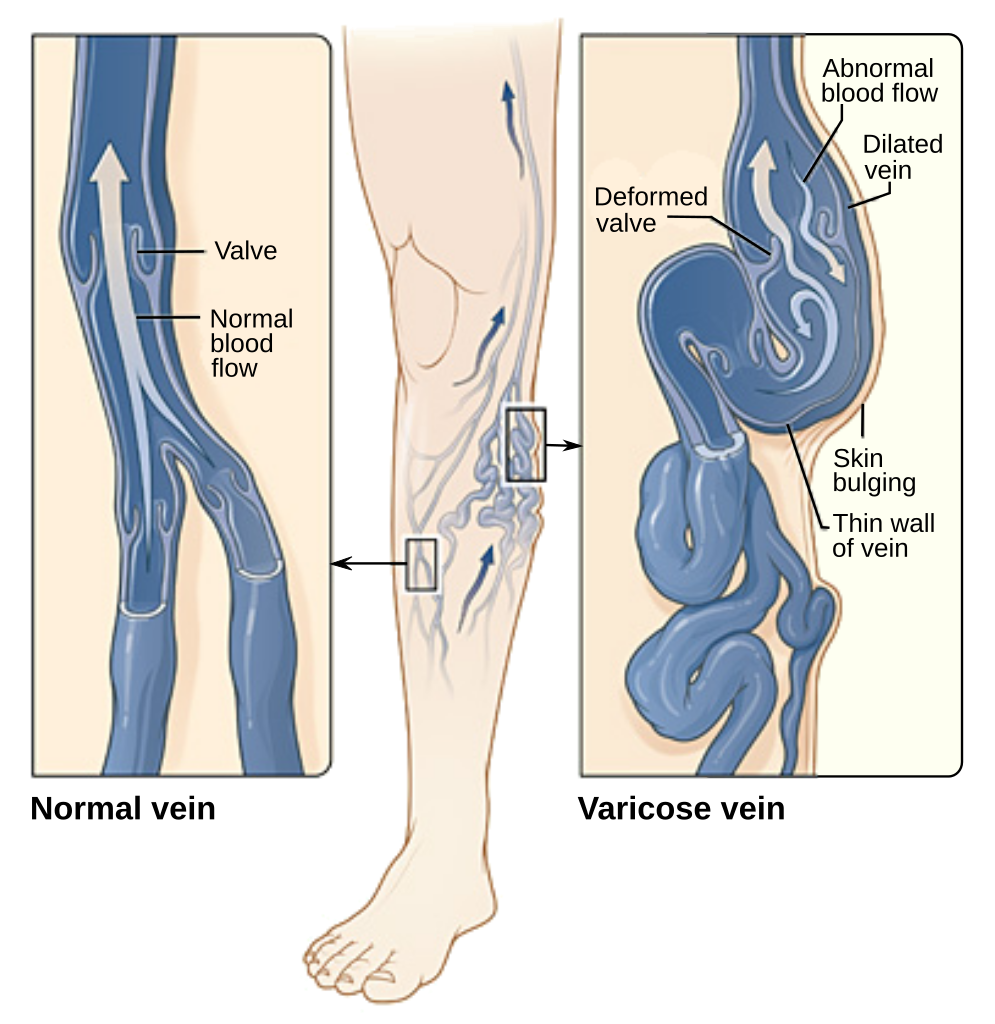
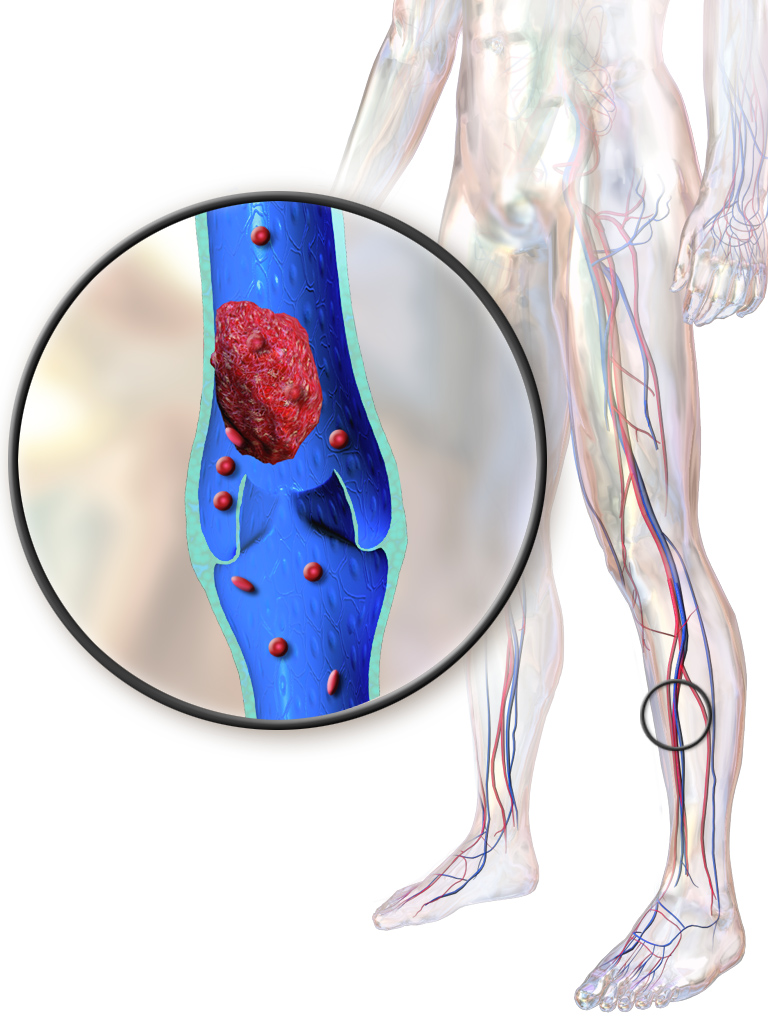
- Deep vein thrombosis (DVT)
A condition which occurs when a blood clot (thrombus) forms in one or more of the deep veins in your body, usually in your legs. Deep vein thrombosis can cause leg pain or swelling, but also can occur with no symptoms. It is a particular hazard of long-haul flying.
- Defecation
The discharge of feces from the body... commonly called pooping.
- Dementia
A chronic or persistent disorder of the mental processes caused by brain disease or injury and marked by memory disorders, personality changes, and impaired reasoning.
- Dendrite
An extension of the cell body of a neuron that receives nerve impulses from other neurons. A neuron will have several dendrites extending from the cell body.
- Dendritic cell
A special type of immune cell that is found in tissues, such as the skin, and boosts immune responses by showing antigens on its surface to other cells of the immune system. A dendritic cell is a type of phagocyte and a type of antigen-presenting cell (APC).
- Denisovan
An extinct species or subspecies of archaic human that ranged across Asia during the Lower and Middle Paleolithic. Denisovans are known from few remains, and, consequently, most of what is known about them comes from DNA evidence.
- Deoxyribose
A sugar derived from ribose by replacing a hydroxyl group with hydrogen.
- Dependence
A state of reliance upon a drug such that when the drug is withdrawn, several physiologic reactions occur.
- Depressant
A type of psychoactive drug that calms the brain, reduces anxious feelings, and induces sleepiness.
- Depression (major depressive disorder)
A common and serious medical illness that negatively affects how you feel, the way you think and how you act. Fortunately, it is also treatable. Depression causes feelings of sadness and/or a loss of interest in activities once enjoyed. It can lead to a variety of emotional and physical problems and can decrease a person’s ability to function at work and at home.
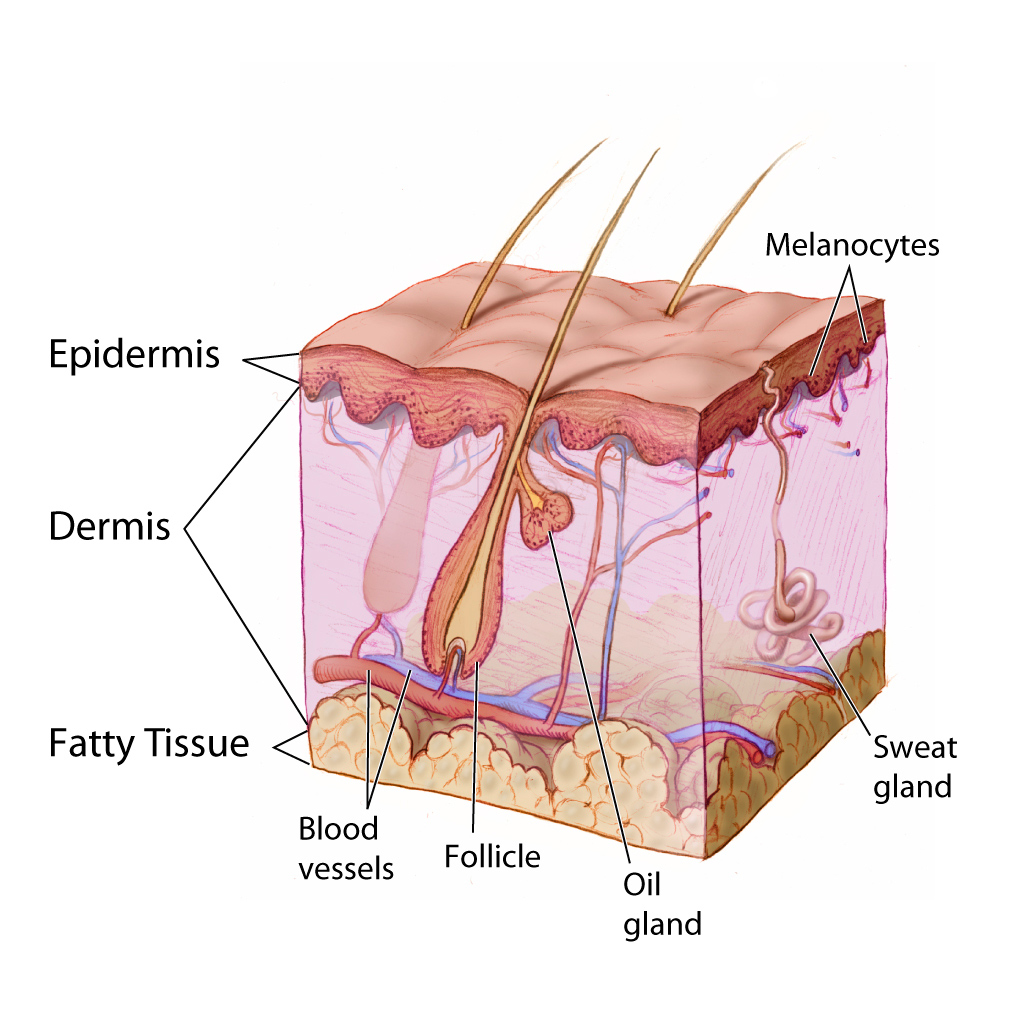
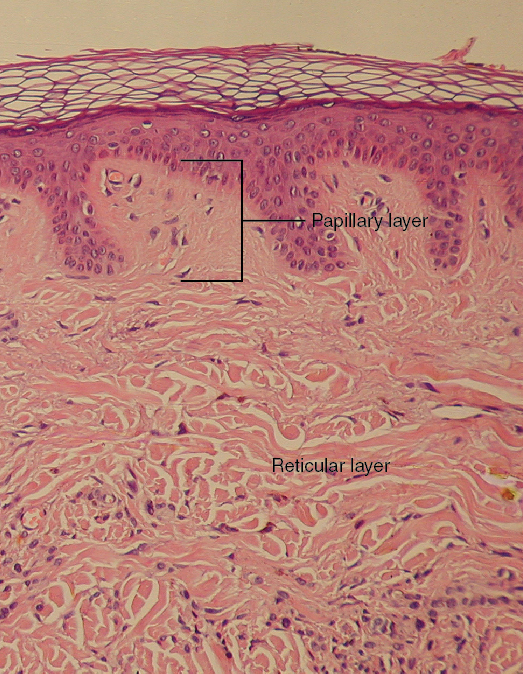
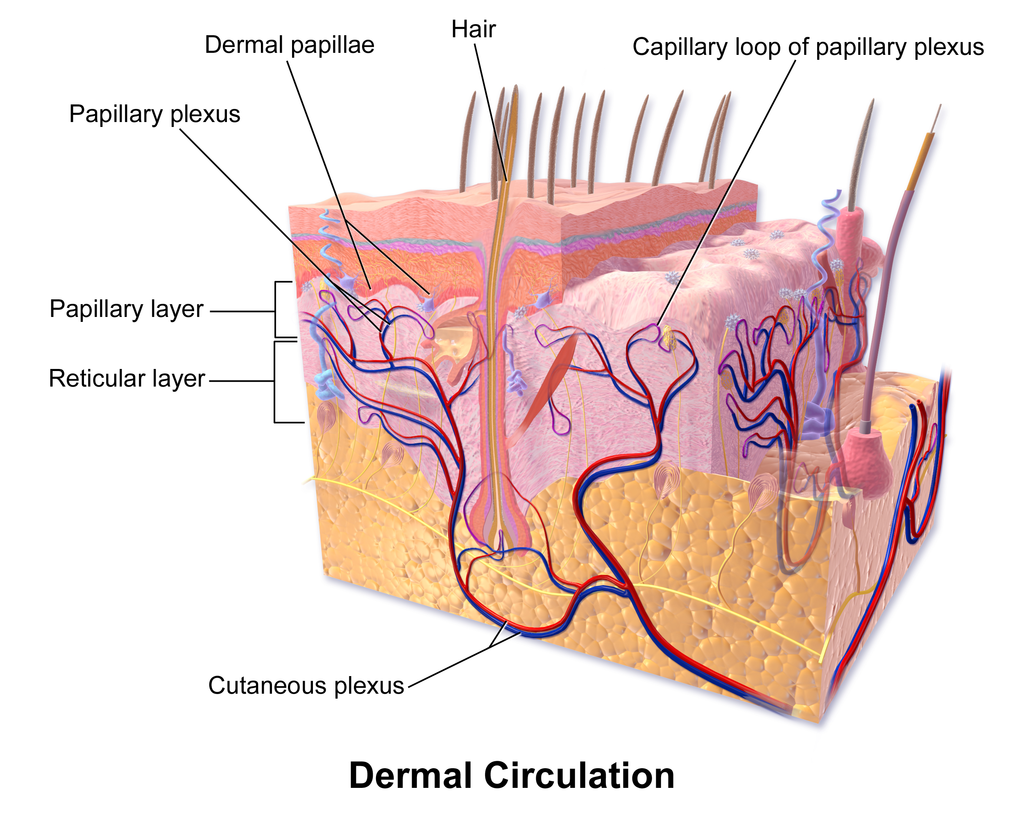
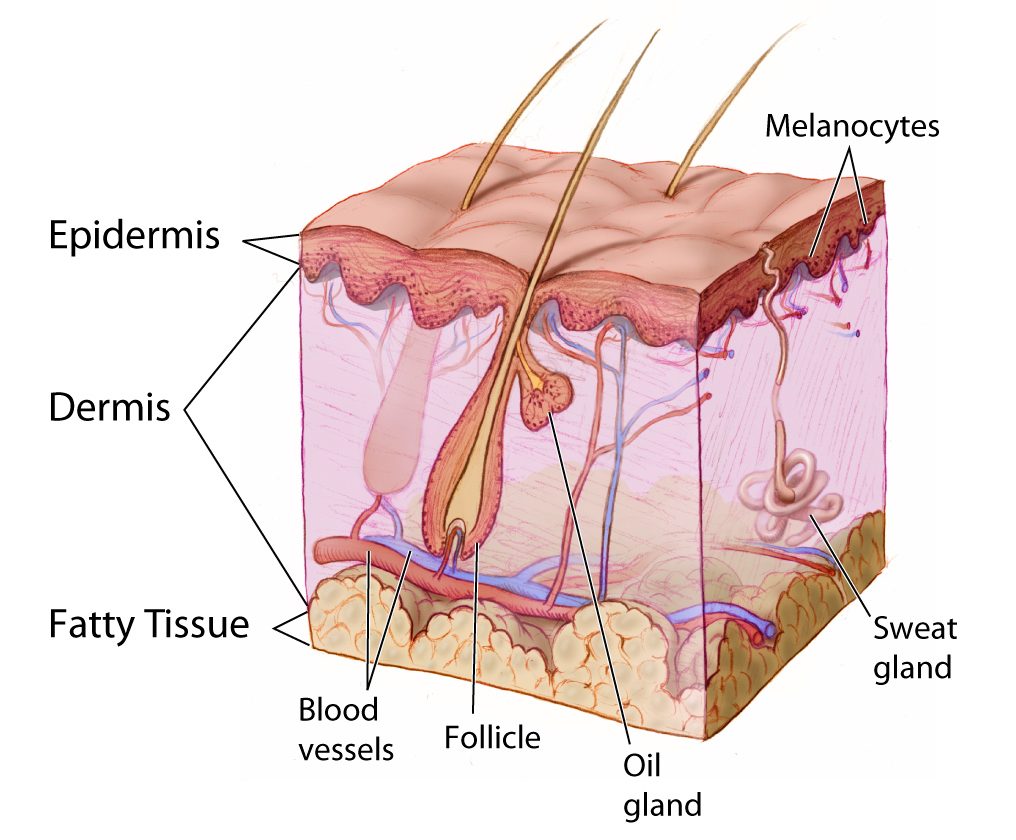
- Dermis
The inner layer of skin that is made of tough connective tissue and contains blood vessels, nerve endings, hair follicles, and glands.
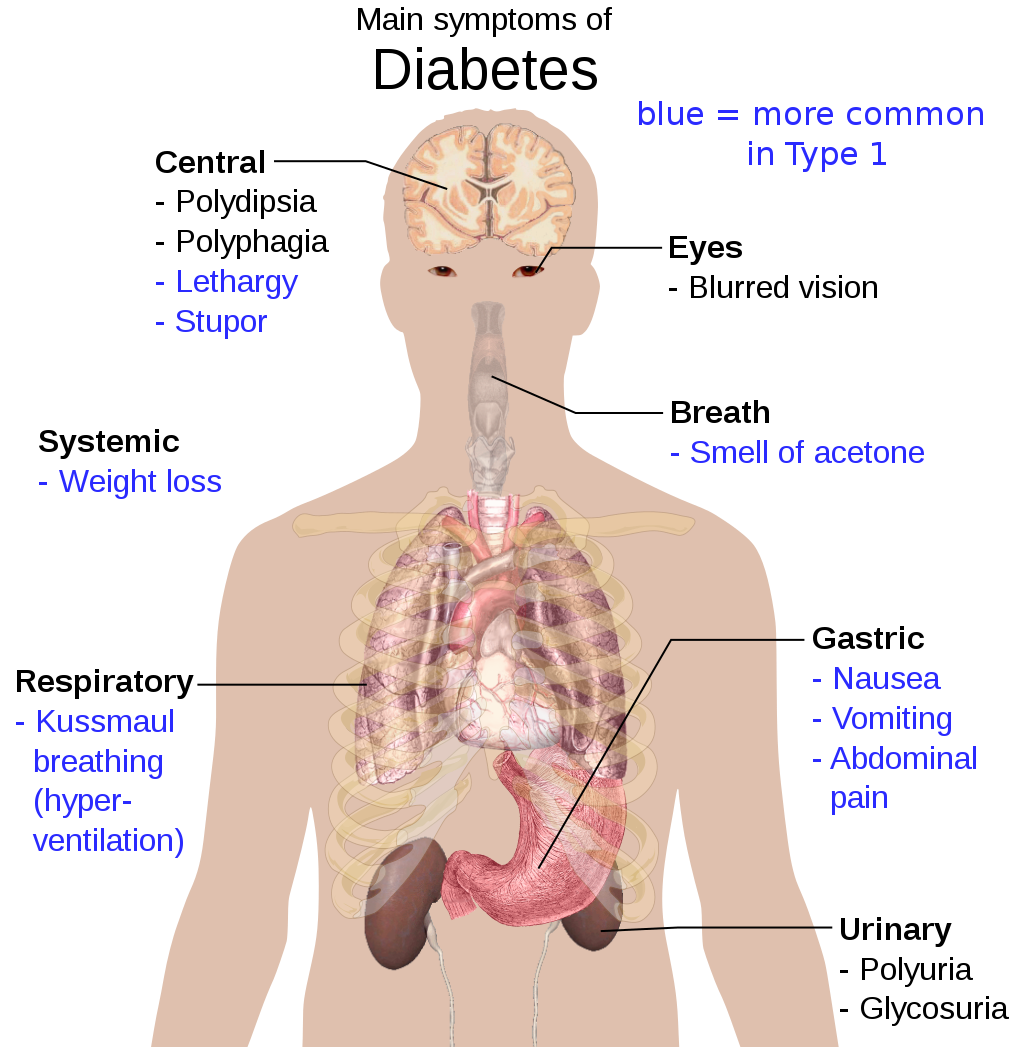
- Diabetes
A disease caused by problems with the pancreatic hormone insulin, which leads to high blood glucose levels and symptoms such as excessive thirst and urination; includes type 1 and type 2 diabetes.
- Diabetes mellitus
A disorder in which blood sugar (glucose) levels are abnormally high because the body does not produce enough insulin to meet its needs.
- Diabetic nephropathy
A progressive kidney disease caused by damage to capillaries in the glomeruli of the kidneys due to poor blood sugar control in people with diabetes.
- Diaphragm (birth control)
A barrier method of birth control. It is a dome-shaped piece of silicon placed over the cervix with spermicide before sex and left in place for at least six hours after sex. Fitting by a healthcare provider is generally required.
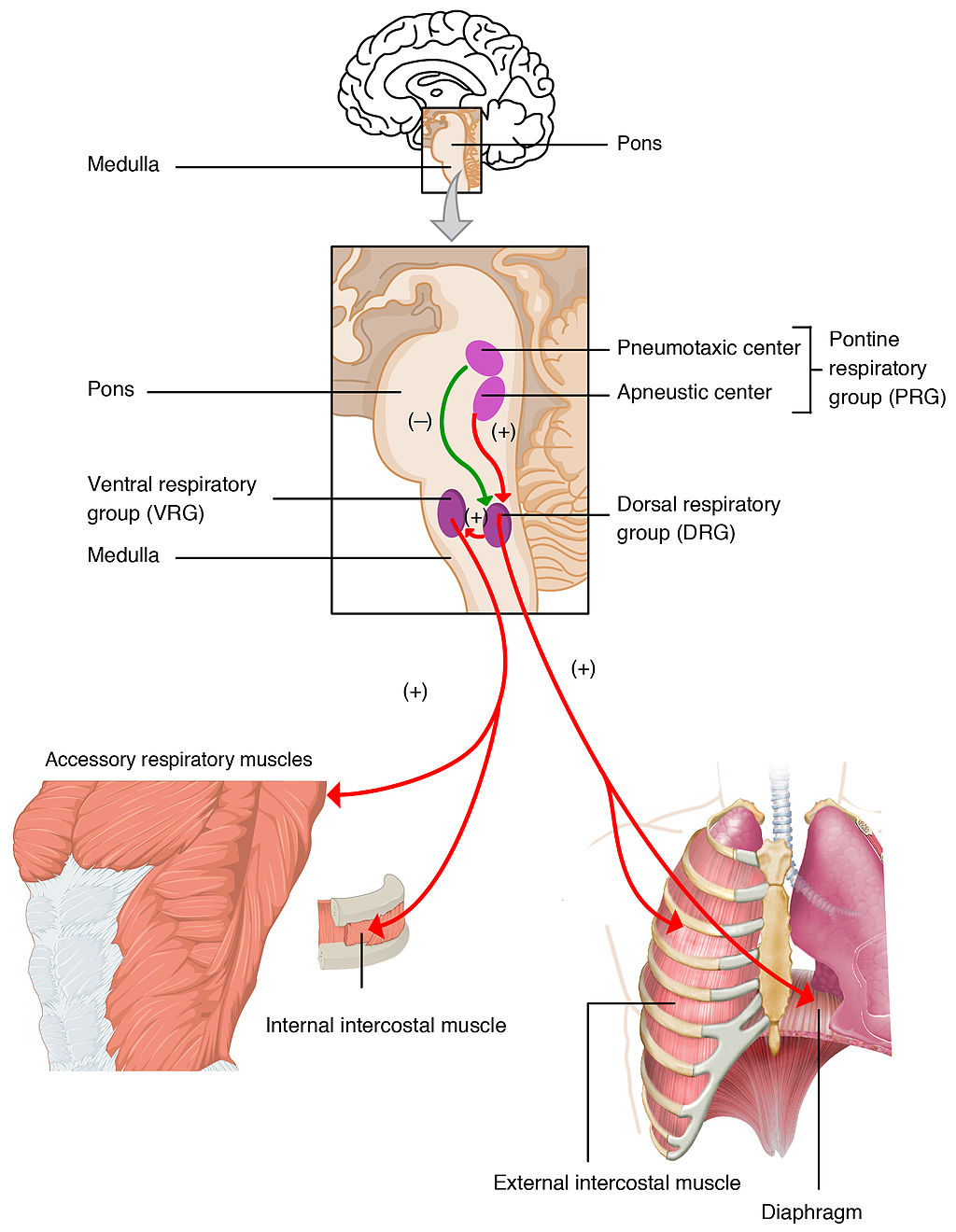
- Diaphragm (breathing muscle)
A large, dome-shaped muscle below the lungs that allows breathing to occur when it alternately contracts and relaxes.
- Diarrhea
A condition in which feces are discharged from the bowels frequently and in a liquid form.
- Diastole
A part of a heartbeat (cardiac cycle) in which the atria contract and pump blood into the ventricles, while the ventricles relax and fill with blood from the atria.
- Diffusion
The movement of a substance from an area of high concentration to an area of low concentration.
- Digestion
The process of breaking down food into nutrients that can be absorbed by blood or lymph.
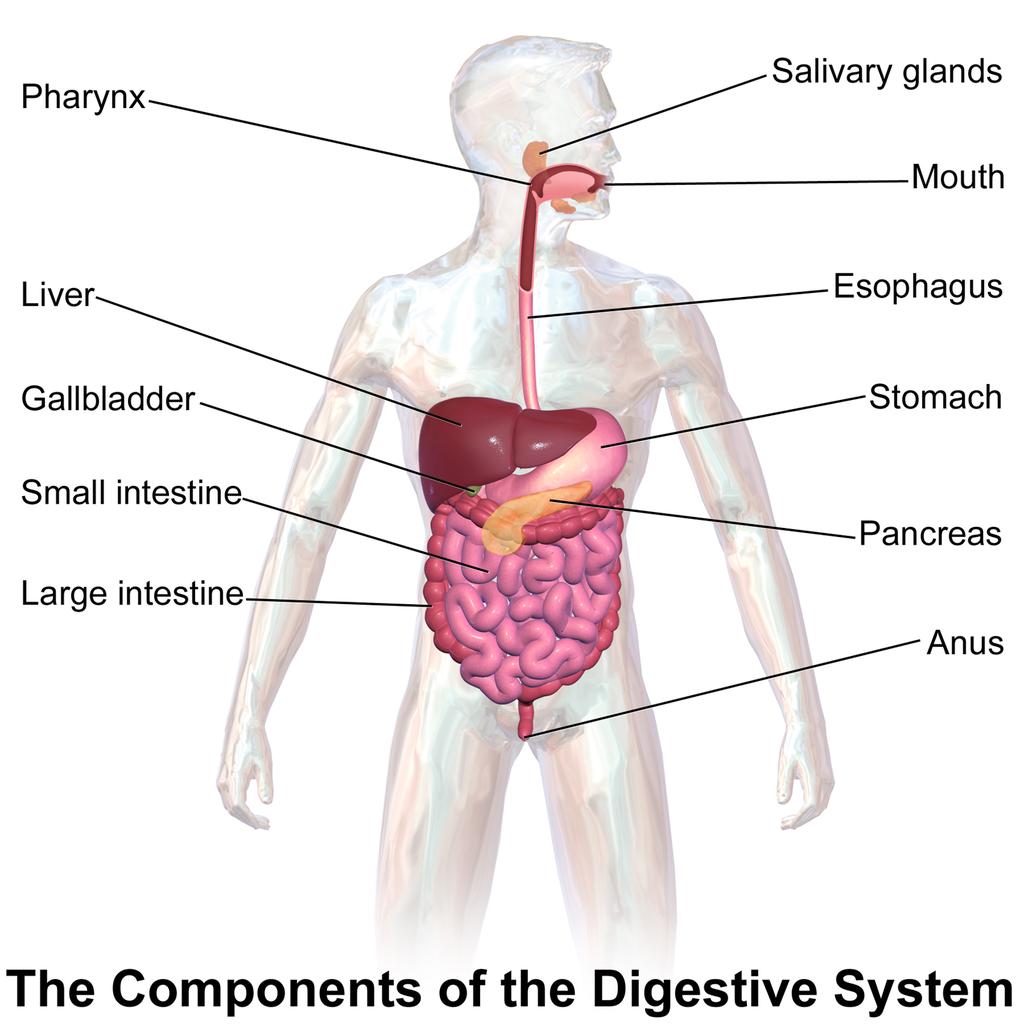
- Digestive system
A body system including a series of hollow organs joined in a long, twisting tube from the mouth to the anus. The hollow organs that make up the GI tract are the mouth, esophagus, stomach, small intestine, large intestine, and anus. The liver, pancreas, and gallbladder are the solid organs of the digestive system.
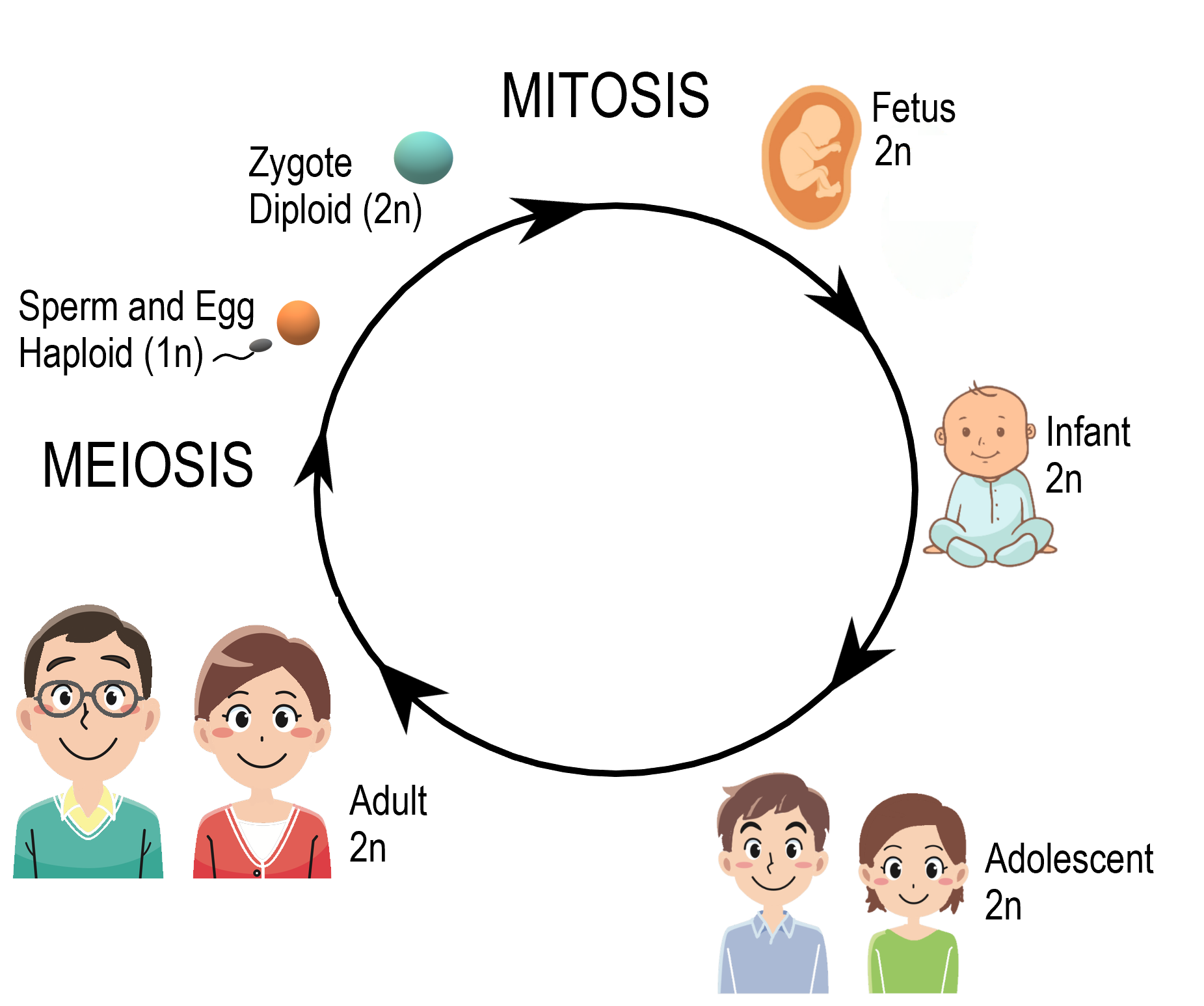
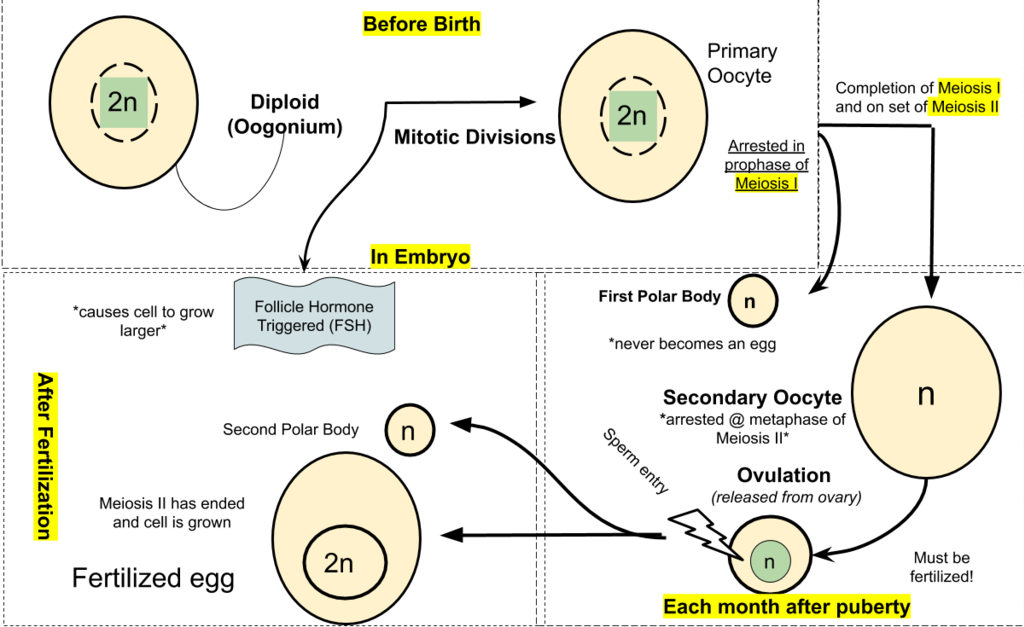
- Diploid
Describes a cell that contain two copies of each chromosome.
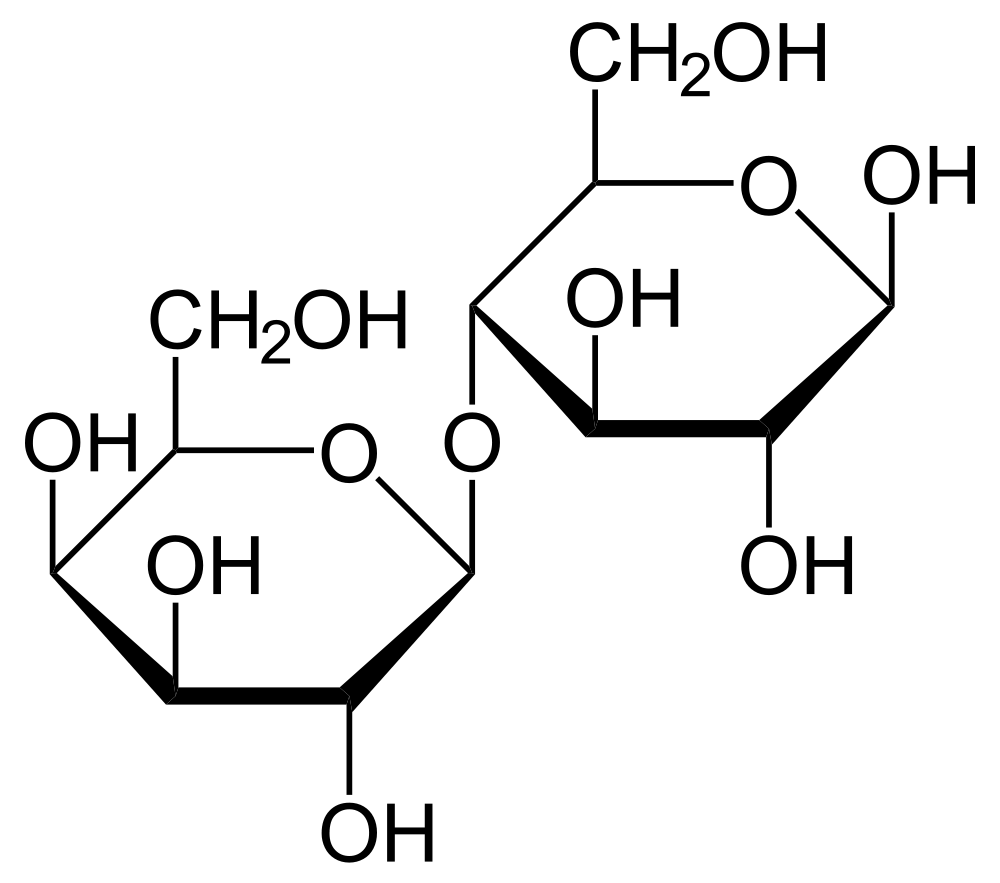
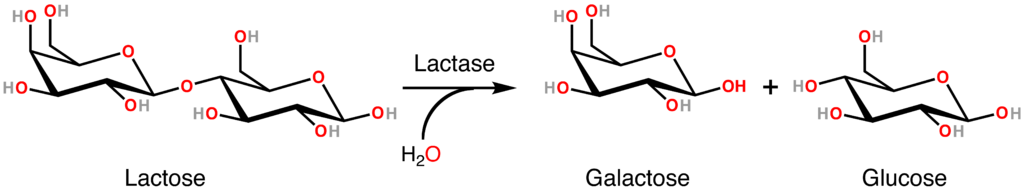
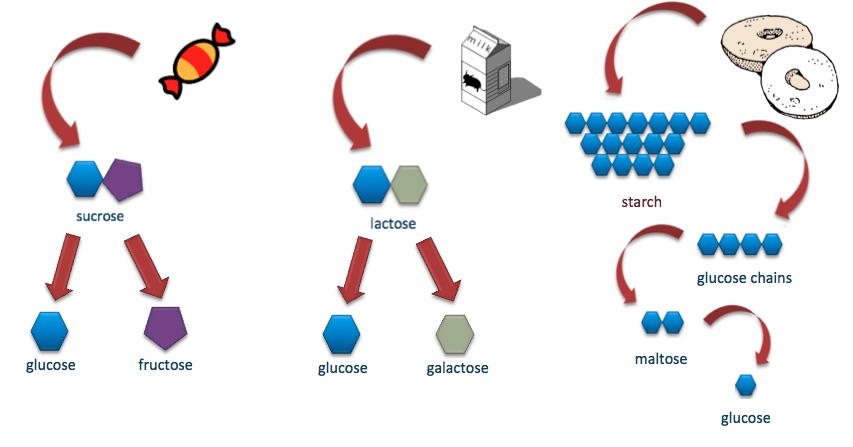
- Disaccharide
The sugar formed when two monosaccharides are joined by glycosidic linkage.
- Distal convoluted tubule
A portion of kidney nephron between the loop of Henle and the collecting tubule. It is the part of the nephron and that is concerned especially with the concentration of urine.
- Diverticulitis
A disease in which one or more pouches (diverticula) in the large intestine become infected and inflamed.
- Diverticulosis
A condition in which pouches called diverticula form in the wall of the large intestine.
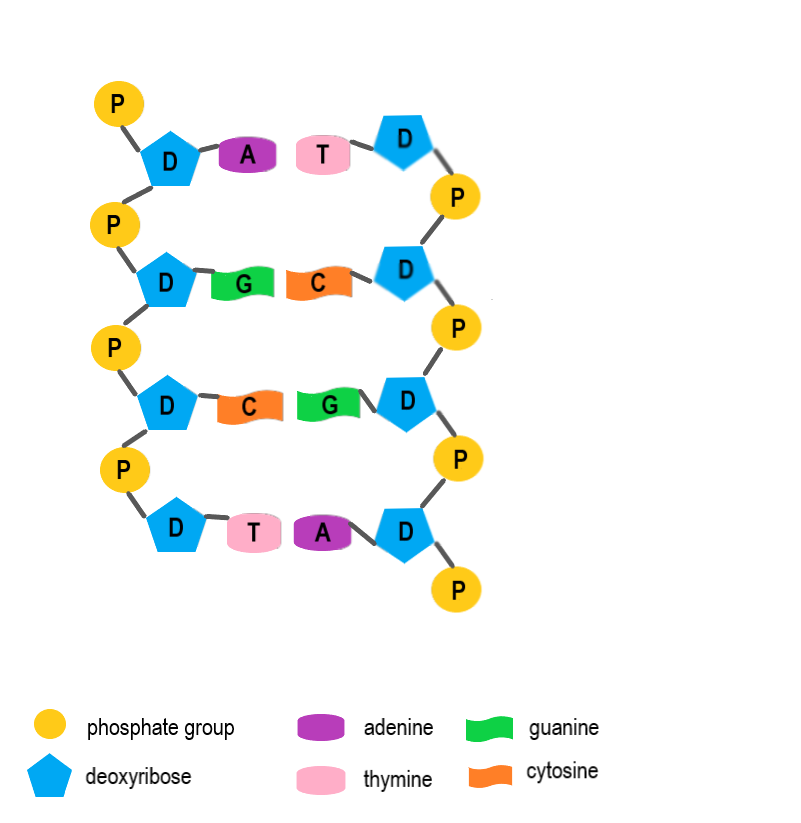
- DNA
Deoxyribonucleic acid - the molecule carrying genetic instructions for the development, functioning, growth and reproduction of all known organisms and many viruses.
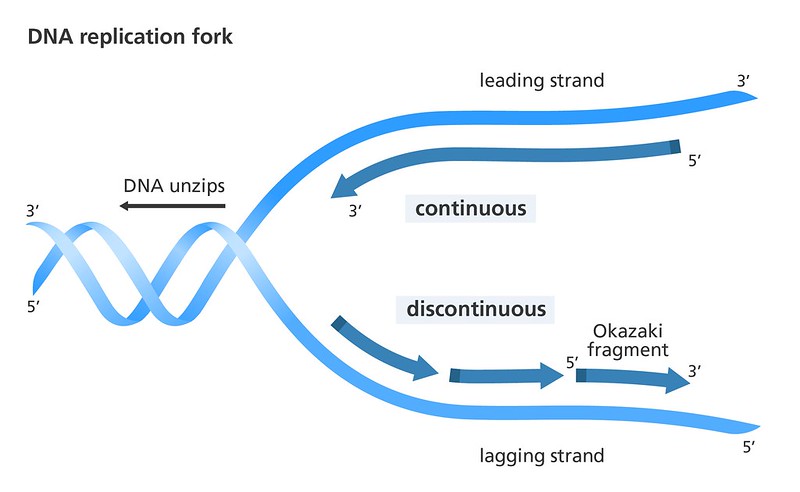
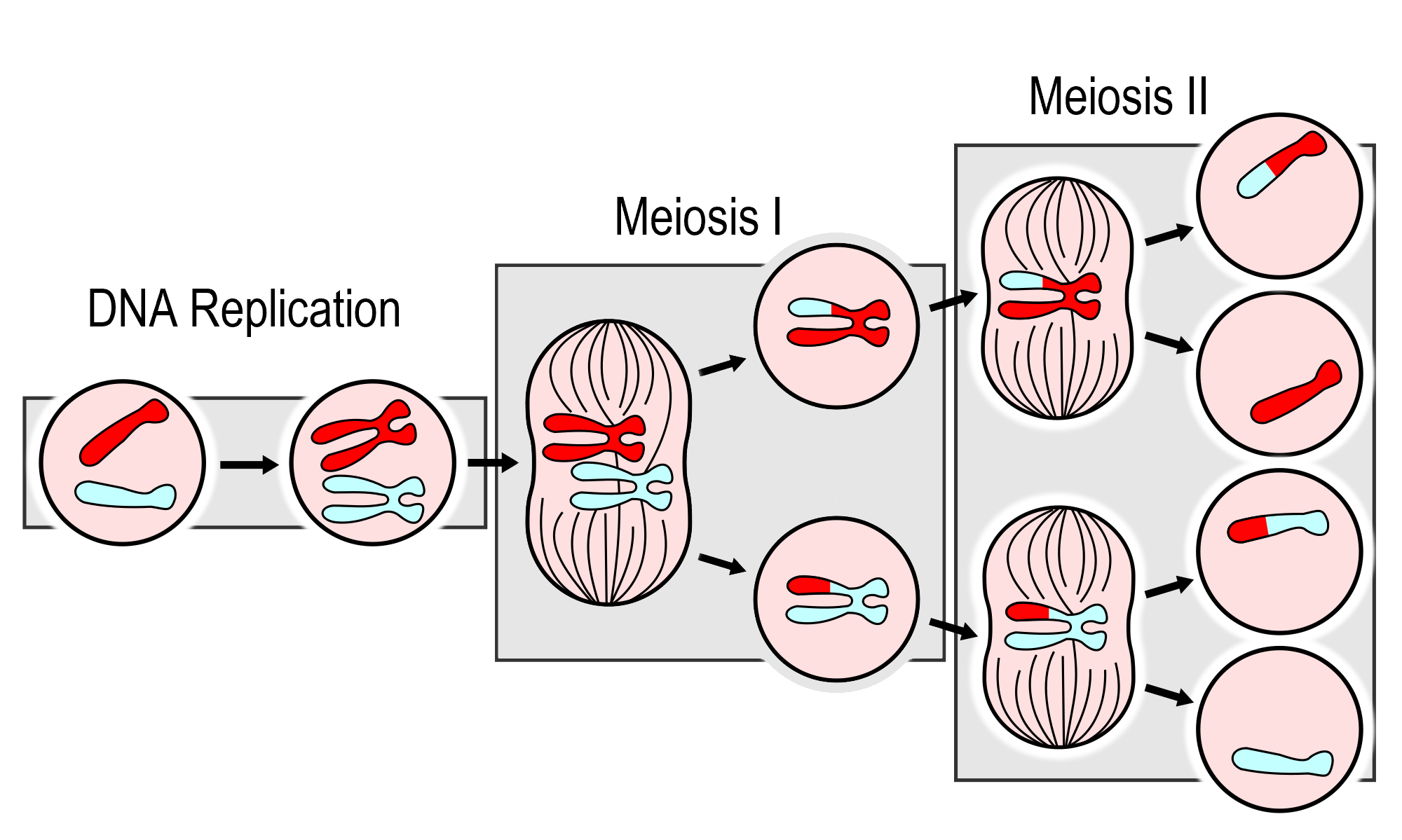
- DNA replication
The process by which DNA is copied.
- Domain
A taxon that is larger and more inclusive than the kingdom.
- Dominance
The phenomenon of one variant of a gene on a chromosome masking or overriding the effect of a different variant of the same gene on the other copy of the chromosome.
- Dominant
Refers to the relationship between two versions of a gene. Individuals receive two versions of each gene, known as alleles, from each parent. If the alleles of a gene are different, one allele will be expressed; it is the dominant gene. The effect of the other allele, called recessive, is masked.
- Dorsal cavity
A major human body cavity that includes the head and the posterior (back) of the trunk and holds the brain and spinal cord.
- Double helix
The shape formed by two parallel lines that twist around each other.
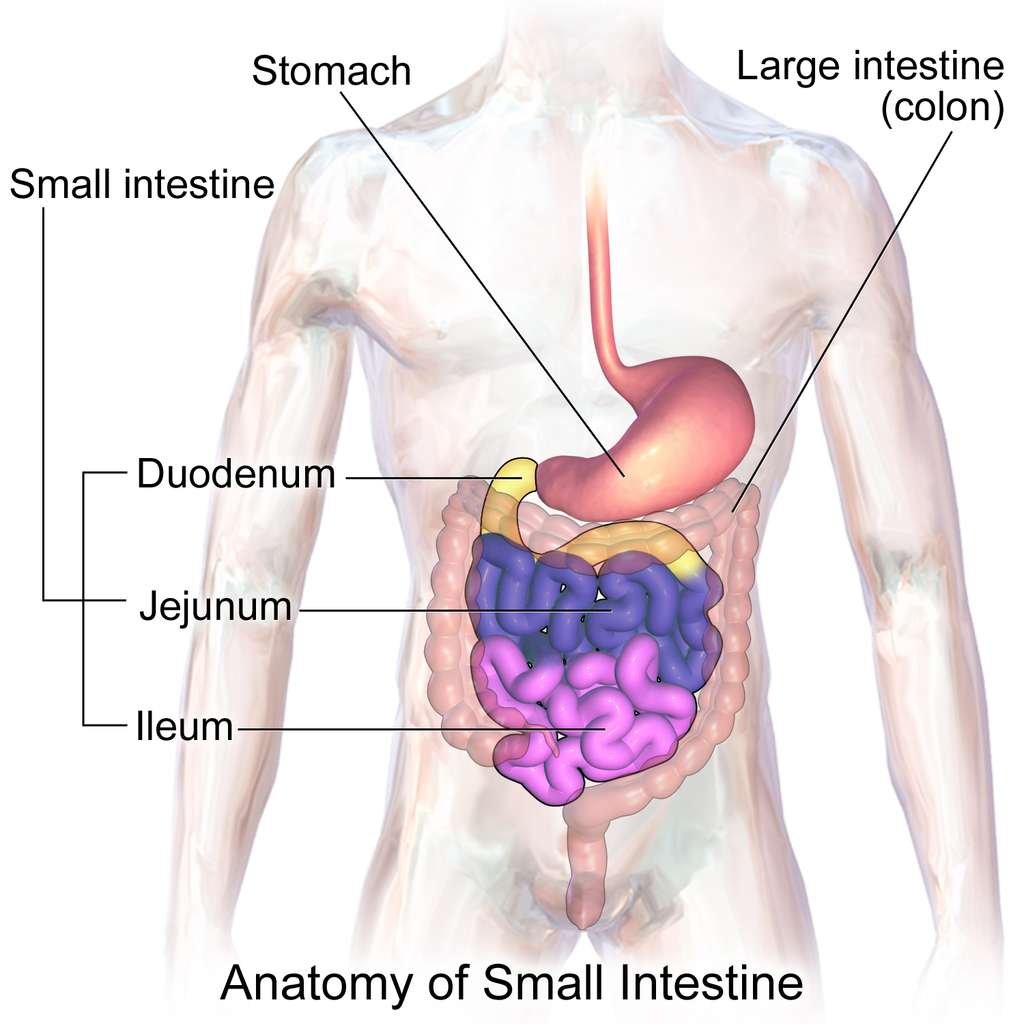
- Duodenum
The first and shortest of three parts of the small intestine where most chemical digestion occurs.
- E-cigarette
An electronic device that simulates tobacco smoking. It consists of an atomizer, a power source such as a battery, and a container such as a cartridge or tank. Instead of cigarette smoke, the user inhales vapor, so using an e-cigarette is called "vaping".
- Ear
A special sensory organ that collects and amplifies sound waves and information on body position and transforms them into nerve impulses that travel to the brain.
- Eccrine sweat gland
The major sweat glands of the human body, sometimes called merocrine glands, found in virtually all skin, with the highest density in palm and soles, then on the head, but much less on the trunk and the extremities.
- Ecosystem
A community of livings things interrelated with their physical and chemical environment.
- Effector
A component of a homeostatic control mechanism, such as a gland or an organ, that acts on a signal from the control center to move the variable back toward the set point.
- Egg or ovum
A mature female reproductive cell, especially of a human or other animal, which can divide to give rise to an embryo usually only after fertilization by a male cell.
- Ejaculation
The process in males in which muscle contractions propel sperm from the epididymes and out through the urethra in semen.
- Ejaculatory duct
One of two tubes in the male reproductive system that joins the vas deferens with the urethra and carries semen during ejaculation.
- Elastin
A key protein of the extracellular matrix. It is highly elastic and present in connective tissue allowing many tissues in the body to resume their shape after stretching or contracting. Elastin helps skin to return to its original position when it is poked or pinched.
- Electrochemical gradient
A gradient of electrochemical potential, usually for an ion that can move across a membrane. The gradient consists of two parts, the chemical gradient, or difference in solute concentration across a membrane, and the electrical gradient, or difference in charge across a membrane.
- Electromagnetic force
A type of physical interaction that occurs between electrically charged particles.
- Electron
A sub-atomic particle with a charge of -1.
- Electron transport
A series of electron transporters embedded in the inner mitochondrial membrane that shuttles electrons from NADH and FADH2 to molecular oxygen. In the process, protons are pumped from the mitochondrial matrix to the intermembrane space, and oxygen is reduced to form water.
- Element
Elements are chemically the simplest substances and hence cannot be broken down using chemical reactions. An element is a substance whose atoms all have the same number of protons.
- Elimination
The process in which wastes pass out of the body.
- Embryo
A stage of growth and development that occurs from implantation in the uterus through the eighth week after fertilization.
- Embryonic
An early stage of development of a multicellular organism. In general, in organisms that reproduce sexually, embryonic development refers to the portion of the life cycle that begins just after fertilization and continues through the formation of body structures, such as tissues and organs.
- Emergency contraception
Any form of birth control that is used after unprotected vaginal intercourse.
- Empathogens
A type of psychoactive drug that produces feelings of empathy with other people.
- Endergonic reaction
A chemical reaction which happens spontaneously and results in the release of energy.
- Endocardium
The thin, smooth membrane which lines the inside of the chambers of the heart and forms the surface of the valves.
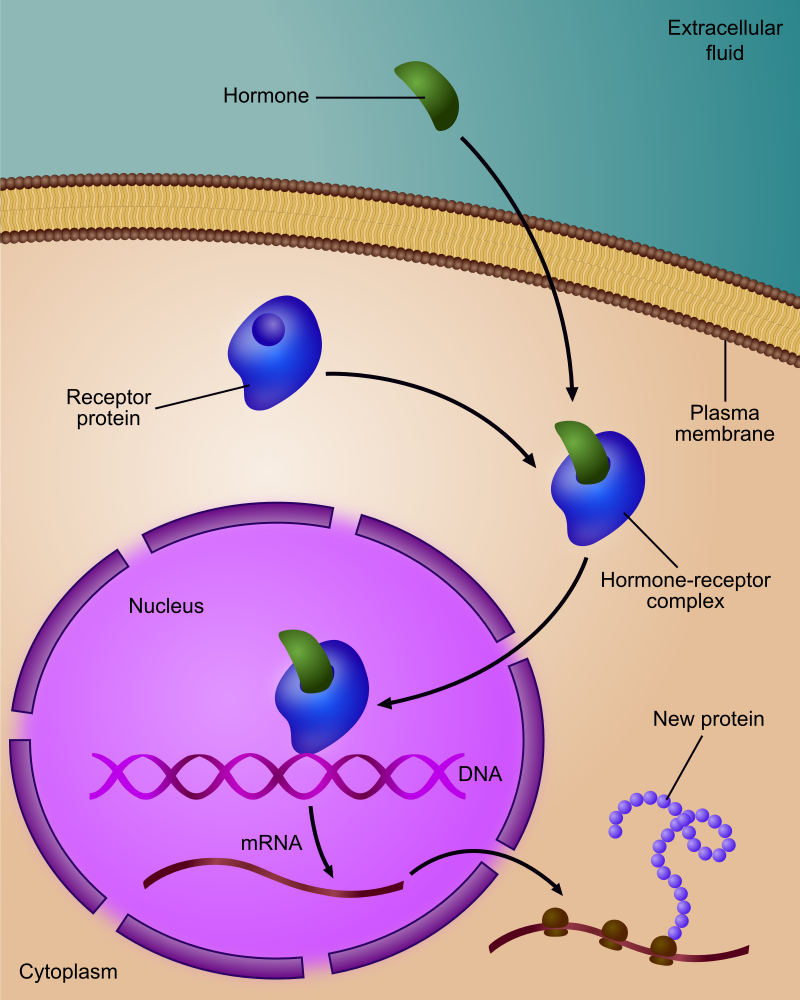
- Endocrine gland
Any gland of the endocrine system, which is the system of glands that releases hormones directly into the blood.
- Endocrine system
The body system which acts as a chemical messenger system comprising feedback loops of the hormones released by internal glands of an organism directly into the circulatory system, regulating distant target organs. In humans, the major endocrine glands are the thyroid gland and the adrenal glands.
- Endocytosis
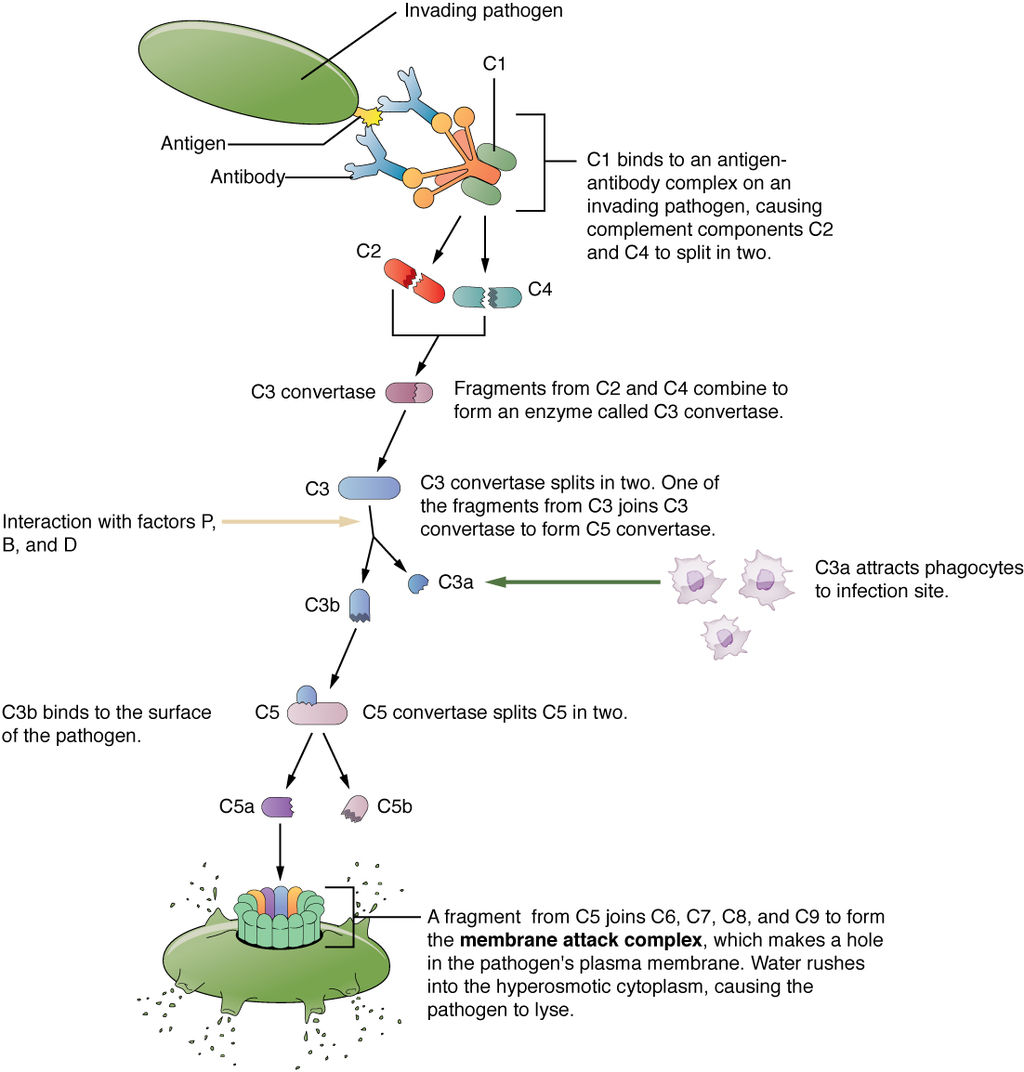
Endocytosis is a cellular process in which substances are brought into the cell. The material to be internalized is surrounded by an area of cell membrane, which then buds off inside the cell to form a vesicle containing the ingested material. Endocytosis includes pinocytosis and phagocytosis.
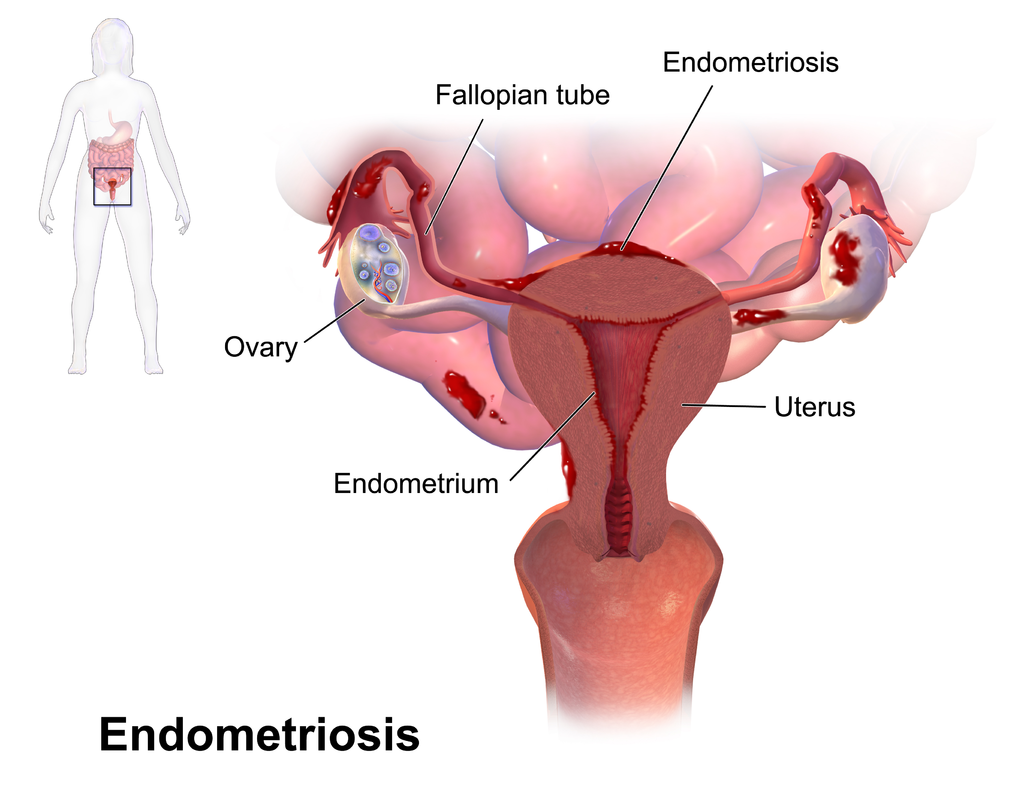
- Endometriosis
A disease in which endometrial tissue grows outside the uterus, typically causing pain and bleeding.
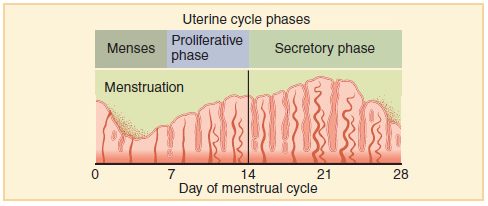
- Endometrium
The innermost layer of the uterus that builds up during each menstrual cycle and helps nourish the embryo if fertilization occurs or is shed from the uterus as menstrual flow if fertilization does not occur.
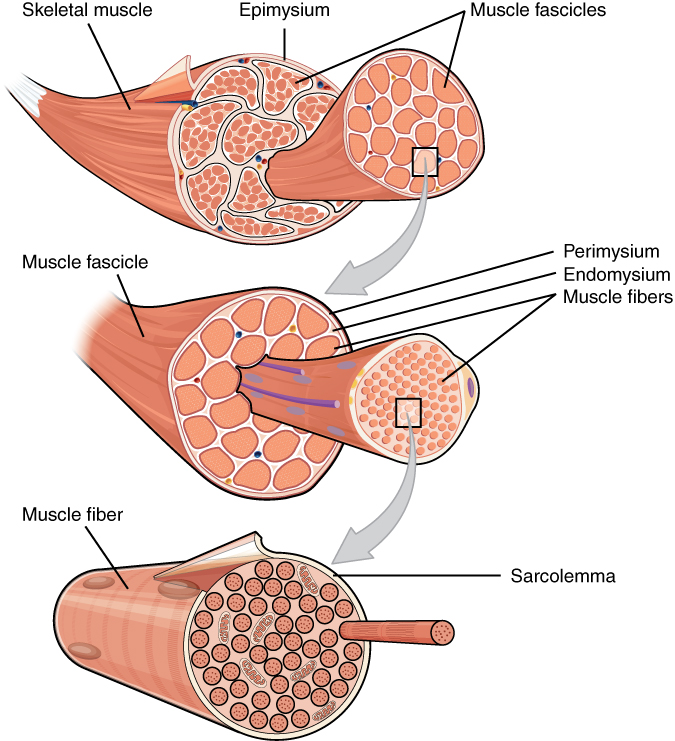
- Endomysium
Meaning within the muscle, is a wispy layer of areolar connective tissue that envelopes each individual muscle fiber. It also contains capillaries, nerves, and lymphatics. It overlies the muscle fiber's cell membrane.
- Endoplasmic reticulum
A network of membranous tubules within the cytoplasm of a eukaryotic cell, continuous with the nuclear membrane. It often has ribosomes attached and is involved in protein and lipid synthesis.
- Endosymbiotic theory
An evolutionary theory of the origin of eukaryotic cells from prokaryotic organisms.
- Endothermic reaction
Any reaction which requires or absorbs energy from its surroundings, usually in the form of heat.
- Energy
The ability to do work.
- Energy coupling
When the energy produced by one reaction or system is used to drive another reaction or system.
- Enhancers
Regulatory DNA sequences that, when bound by specific proteins called transcription factors, enhance the transcription of an associated gene.
- Enteric division
A division of the autonomic nervous system that controls digestive functions.
- Environmental stress
Any physical, chemical, and biological constraints on the productivity of species and on the development of ecosystems.
- Enzyme
Biological molecules that lower amount the energy required for a reaction to occur.
- Eosinophil
A type of immune cell that has granules (small particles) with enzymes that are released during infections, allergic reactions, and asthma. An eosinophil is a type of white blood cell and a type of granulocyte.
- Epidermis
The outer layer of skin that consists mainly of epithelial cells and lacks nerve endings, blood vessels, and other structures.
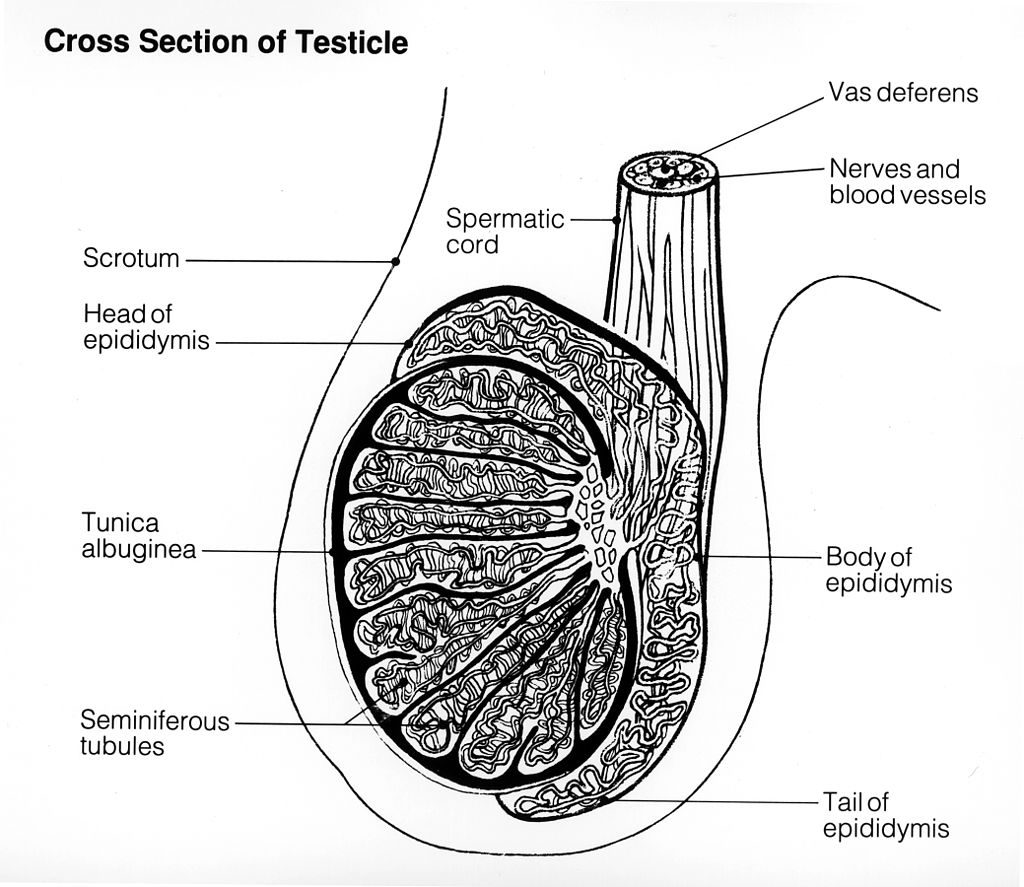
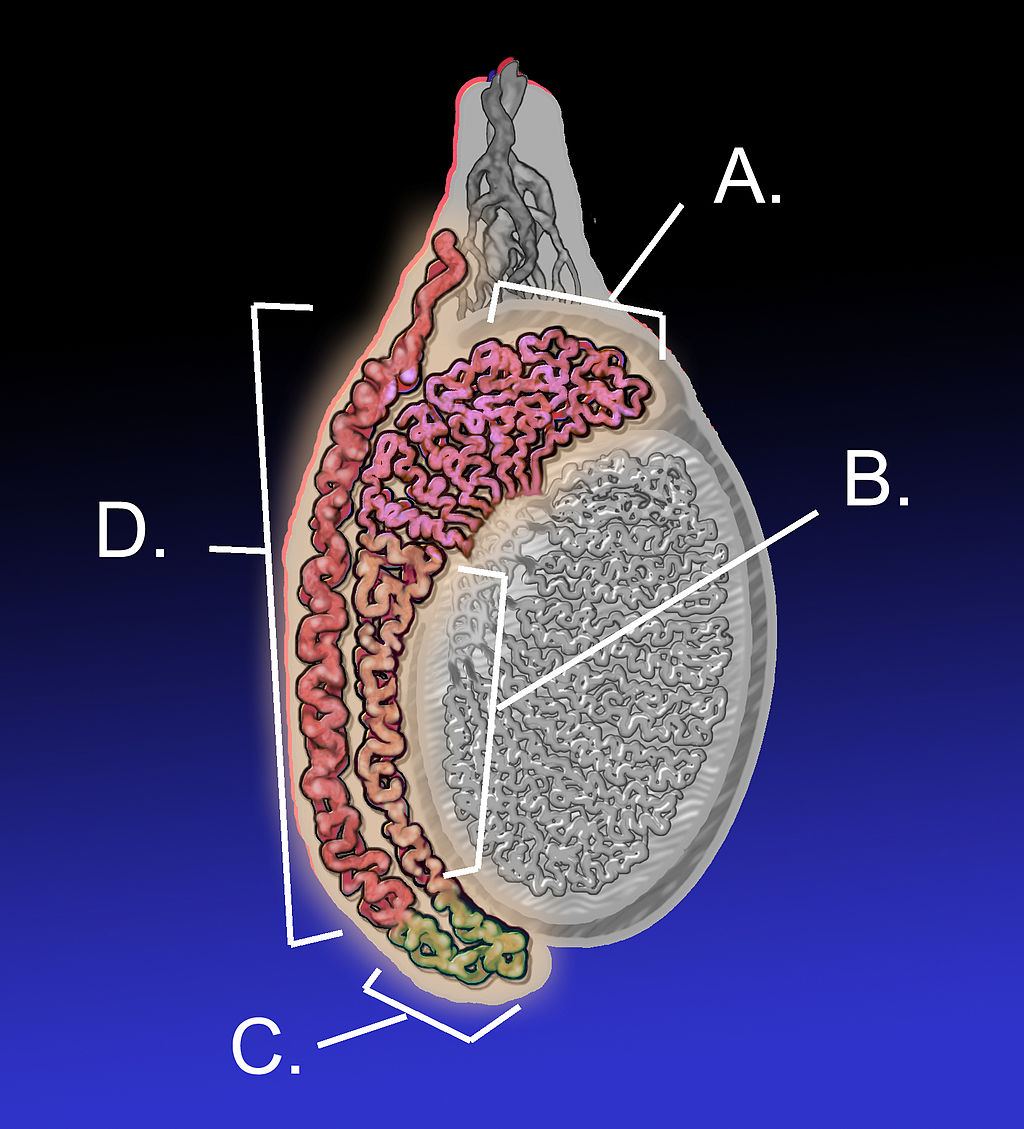
- Epididymis (plural, epididymes)
One of two male reproductive organs where sperm mature and are stored until they leave the body during ejaculation.
- Epididymitis
inflammation of the epididymis, which may be acute or chronic
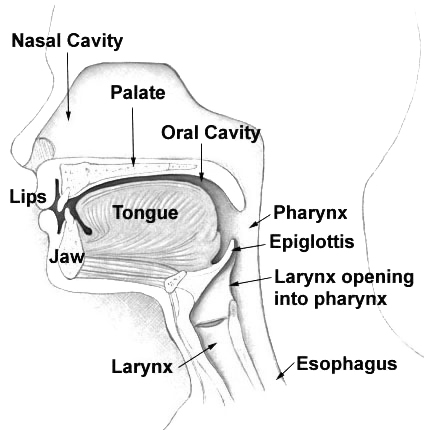
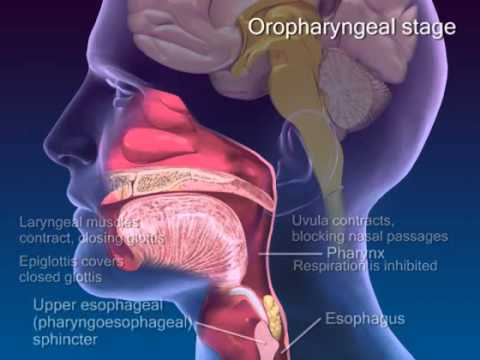
- Epiglottis
A flap of cartilage at the root of the tongue, which is depressed during swallowing to cover the opening of the windpipe.
- Epimysium
A sheath of fibrous elastic tissue surrounding a muscle.
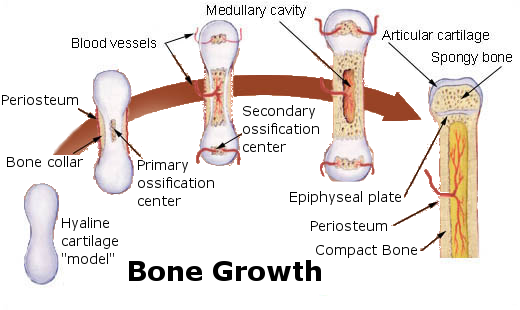
- Epiphyseal plate
Also known as the growth plate, a thin layer of cartilage that lies between the epiphyses and metaphyses, where the growth of long bones takes place.
- Epistasis
A phenomenon in genetics in which the effect of a gene mutation is dependent on the presence or absence of mutations in one or more other genes, respectively termed modifier genes. In other words, the effect of the mutation is dependent on the genetic background in which it appears.
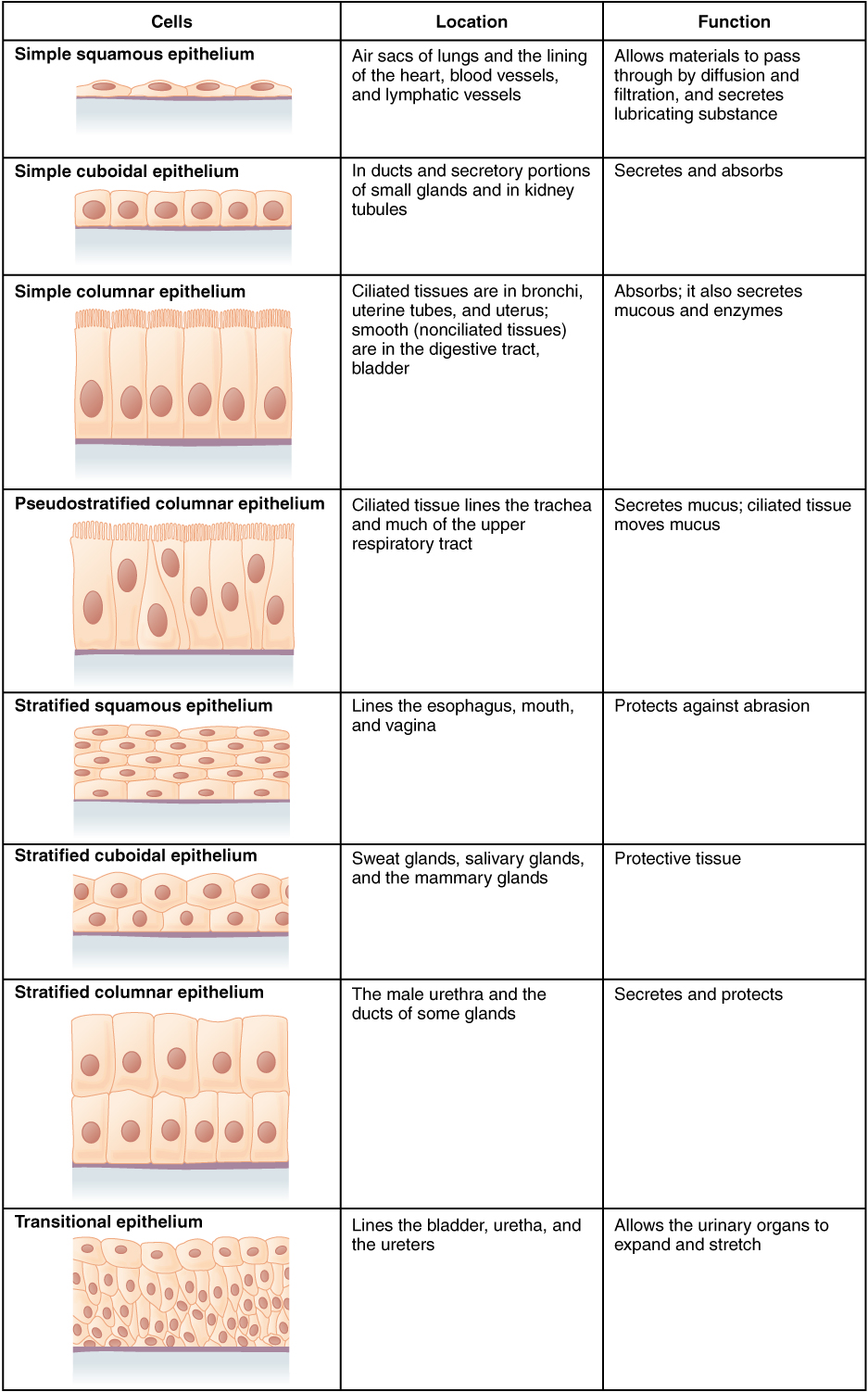
- Epithelial tissue
Tissue which lines the outer surfaces of organs and blood vessels throughout the body, as well as the inner surfaces of cavities in many internal organs. An example is the epidermis, the outermost layer of the skin. There are three principal shapes of epithelial cell: squamous, columnar, and cuboidal.
- Erectile dysfunction
A disorder characterized by the regular and repeated inability of a sexually mature male to obtain or maintain an erection of the penis.
- Erection
A state in which the penis becomes stiff and erect, usually during sexual arousal, as its columns of spongy tissue become engorged with blood.
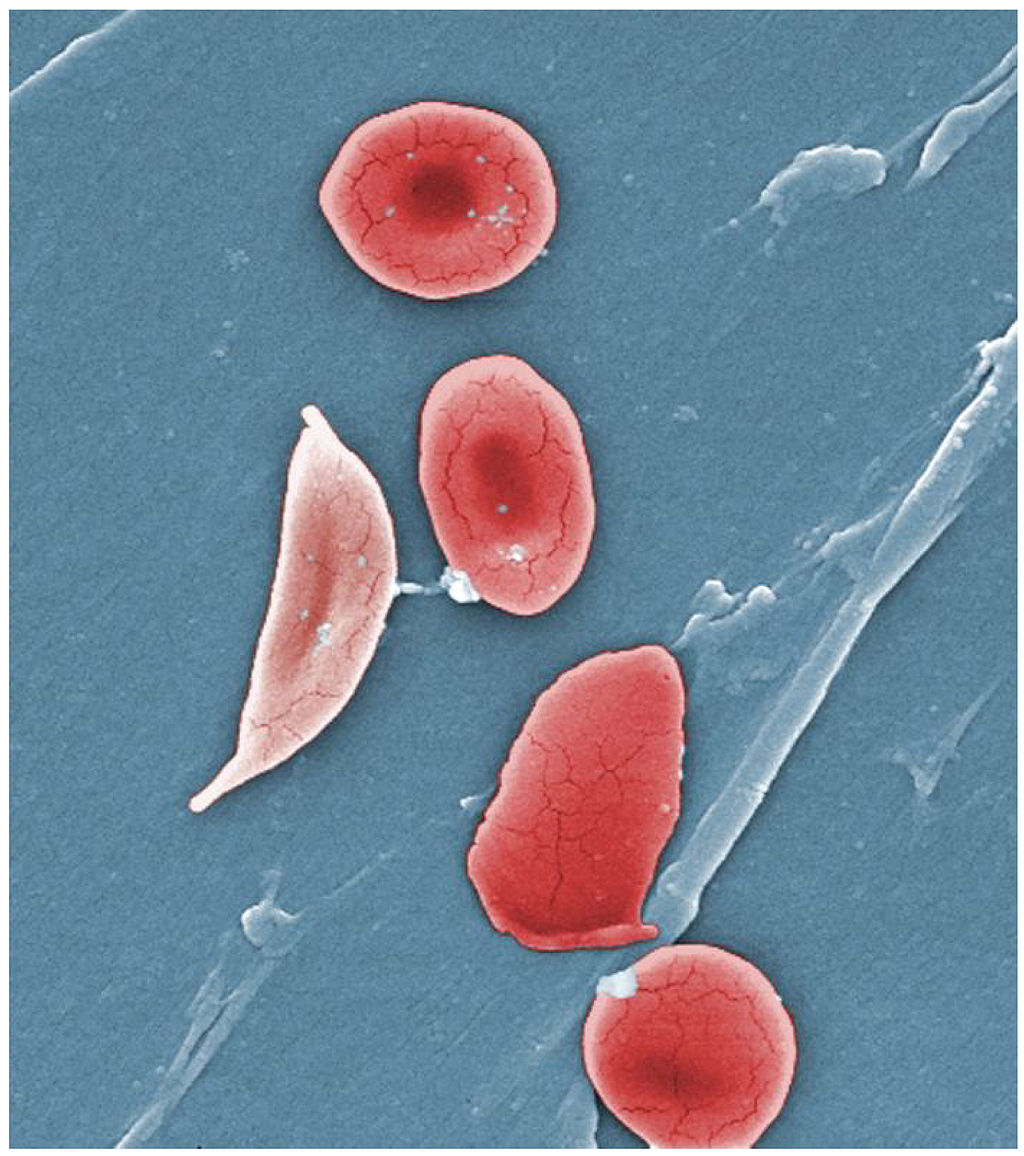
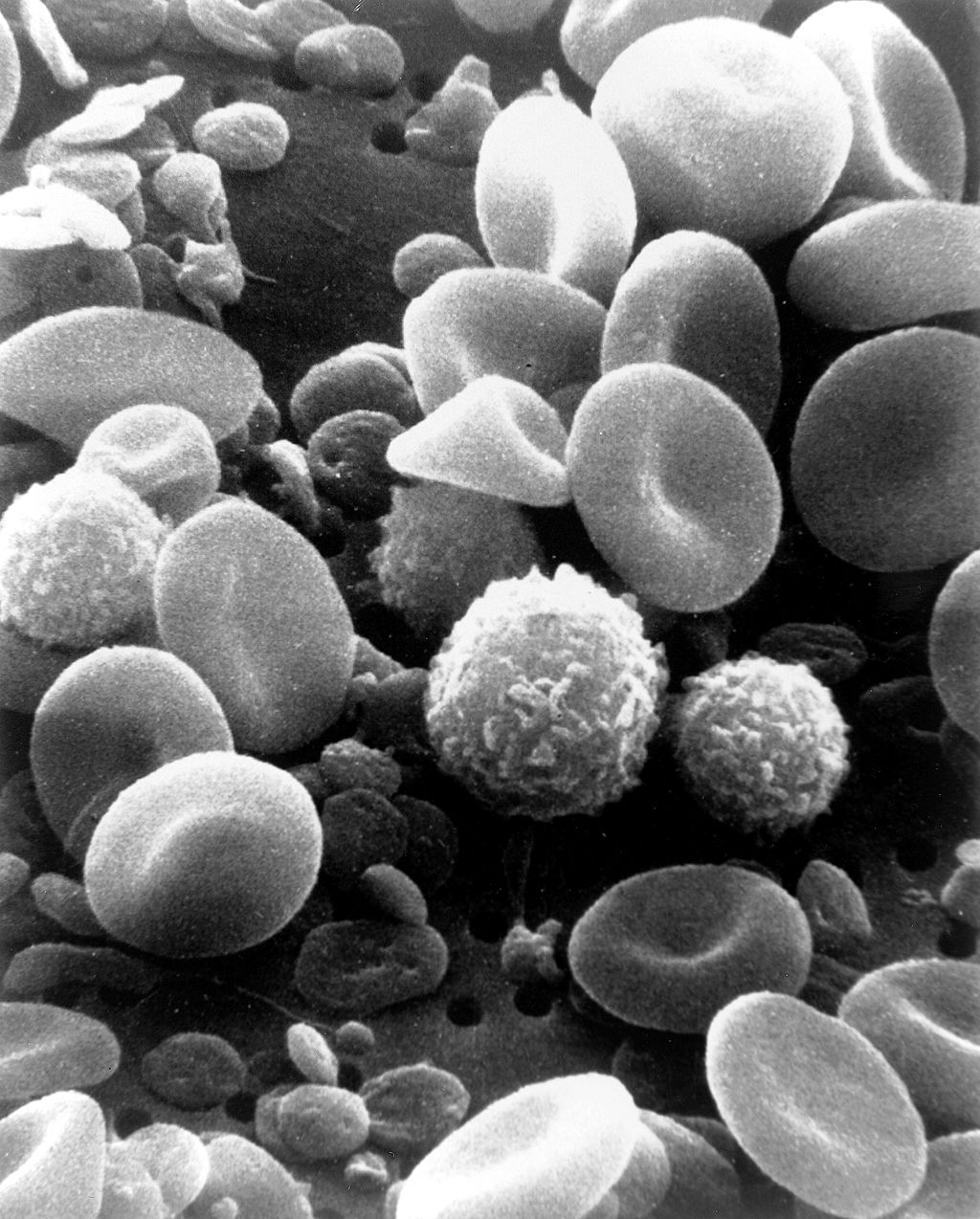
- Erythrocyte
A red blood cell that (in humans) is typically a biconcave disc without a nucleus. Erythrocytes contain the pigment hemoglobin, which imparts the red color to blood, and transport oxygen and carbon dioxide to and from the tissues.
- Erythropoietin
A hormone secreted by the kidneys that increases the rate of production of red blood cells in response to falling levels of oxygen in the tissues.
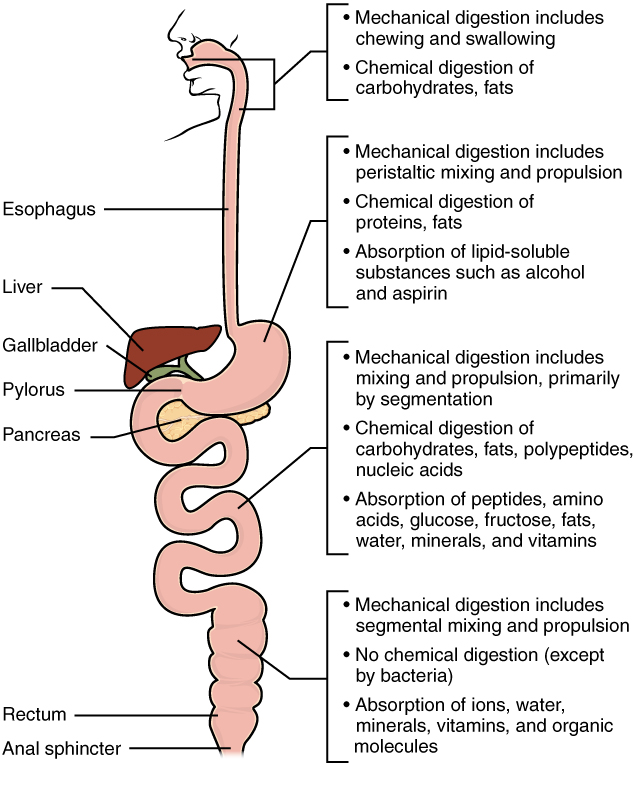
- Esophagus
A long, narrow, tube-like digestive organ through which food passes from the pharynx to the stomach.
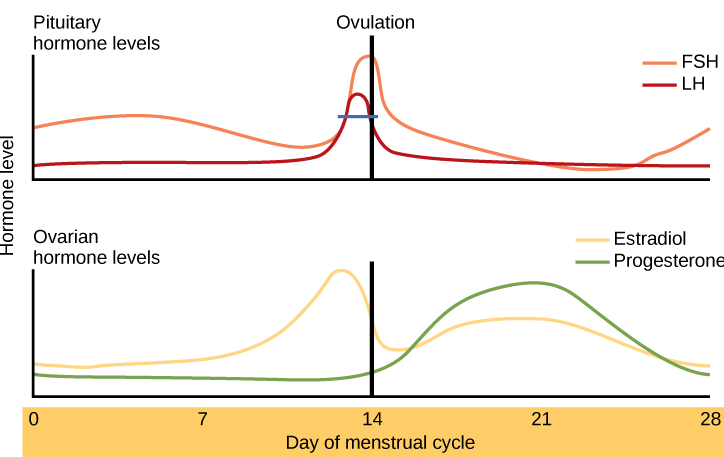
- Estrogen
The female sex hormone secreted mainly by the ovaries.
- Eukaryotic
Cells which have a nucleus enclosed within membranes, unlike prokaryotes, which have no membrane-bound organelles.
- Eumelanin
A dark pigment that predominates in black and brunette hair. There are two different types of eumelanin (brown eumelanin and black eumelanin). A small amount of brown eumelanin in the absence of other pigments causes blond hair.
- Euphoriant
A type of drug that tends to induce a feeling or state of intense excitement and happiness.
- Evidence
The available body of facts or information indicating whether a belief or proposition is true or valid.
- Evolution
The change in characteristics of a population over several generations.
- Evolutionary theory
A theory of evolution by natural selection, first formulated in Darwin's book "On the Origin of Species" in 1859, is the process by which organisms change over time as a result of changes in heritable physical or behavioral traits.
- Excitatory neurotransmitter
A neurotransmitter that will have excitatory effects on the neuron, meaning it will increase the likelihood that a neuron will fire an action potential.
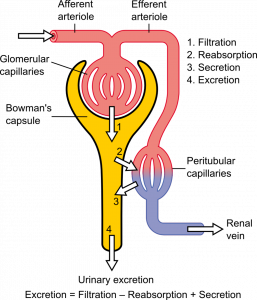
- Excretion
The process of removing wastes and excess water from the body.
- Excretory system
The body system responsible for the elimination of wastes produced by homeostasis. There are several parts of the body that are involved in this process, such as sweat glands, the liver, the lungs and the kidney system. ... From there, urine is expelled through the urethra and out of the body.
- Exergonic reactions
A specific type of exothermic reaction which not only releases energy, but also occurs spontaneously.
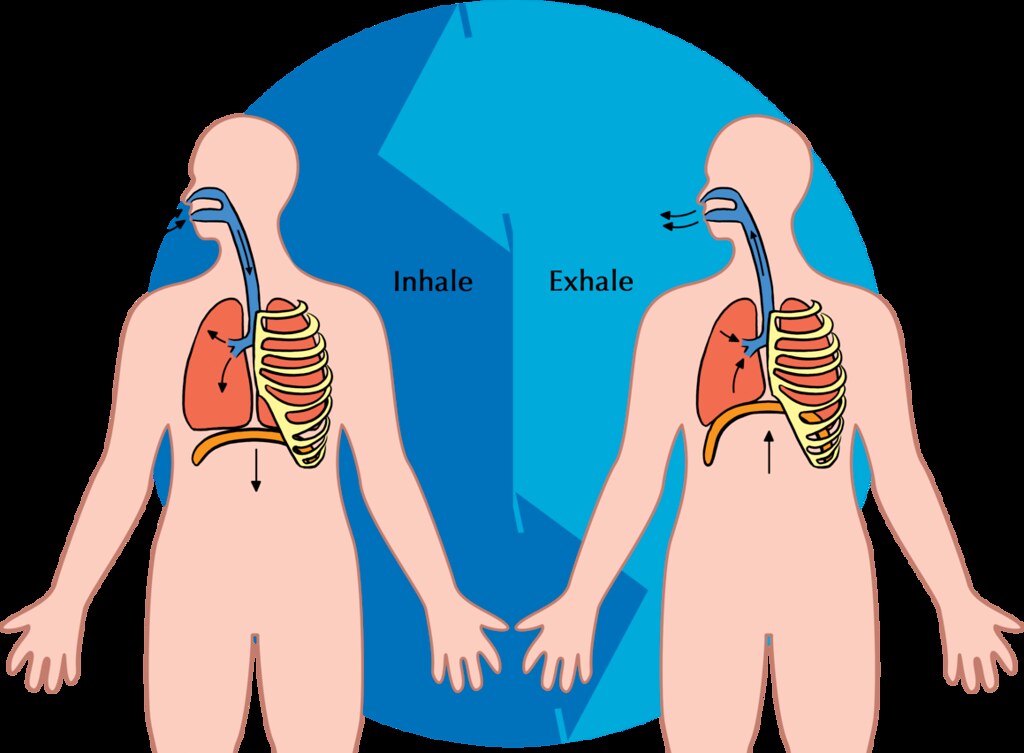
- Exhalation
The action of exhaling or breathing out. Exhalation happens when air or other gases exit the lungs.
- Exocrine gland
Gland such as a sweat gland, salivary gland, or mammary gland that secretes a substance into a duct that carries the secretion to the outside of the body.
- Exocytosis
An important process of plant and animal cells as it performs the opposite function of endocytosis. In exocytosis, membrane-bound vesicles containing cellular molecules are transported to the cell membrane and released into the area surrounding the cell.
- Exothermic reaction
A chemical reaction that releases energy through light or heat.
- Exothermic reactions
A three-dimensional network of extracellular macromolecules, such as collagen, enzymes, and glycoproteins, that provide structural and biochemical support to surrounding cells.
- Eyebrow
The strip of hair growing on the ridge above a person's eye socket.
- Eyelash
Each of the short curved hairs growing on the edges of the eyelids, serving to protect the eyes from dust particles.
- Facilitated diffusion
The passive movement of molecules across the cell membrane with the aid of a membrane protein.
- Falsifiable
In the philosophy of science, falsifiability or refutability is the capacity for a statement, theory or hypothesis to be contradicted by evidence. For example, the statement "All swans are white" is falsifiable because one can observe the existence of black swans.
- Fast-twitch muscle fibers
A type of skeletal muscle cell that is mainly responsible for anaerobic activities such as weight lifting.
- Fatty acids
Long chains of hydrocarbons with a carboxyl group and a methyl group at opposite ends. Can be either saturated, containing mostly single bonds between adjacent carbons, or unsaturated, containing many double bonds between adjacent carbons.
- Feces
Solid waste that remains after food is digested and that is eliminated from the body through the anus.
- Feedback mechanism
A loop system wherein the system responds to a perturbation. The response may be in the same direction (as in positive feedback) or in the opposite direction (as in negative feedback). A feedback mechanism may be observed at the level of cells, organisms, ecosystems, or the biosphere.
- Fermentation
A metabolic process that produces chemical changes in organic substrates through the action of enzymes. In biochemistry, it is narrowly defined as the extraction of energy from carbohydrates in the absence of oxygen.
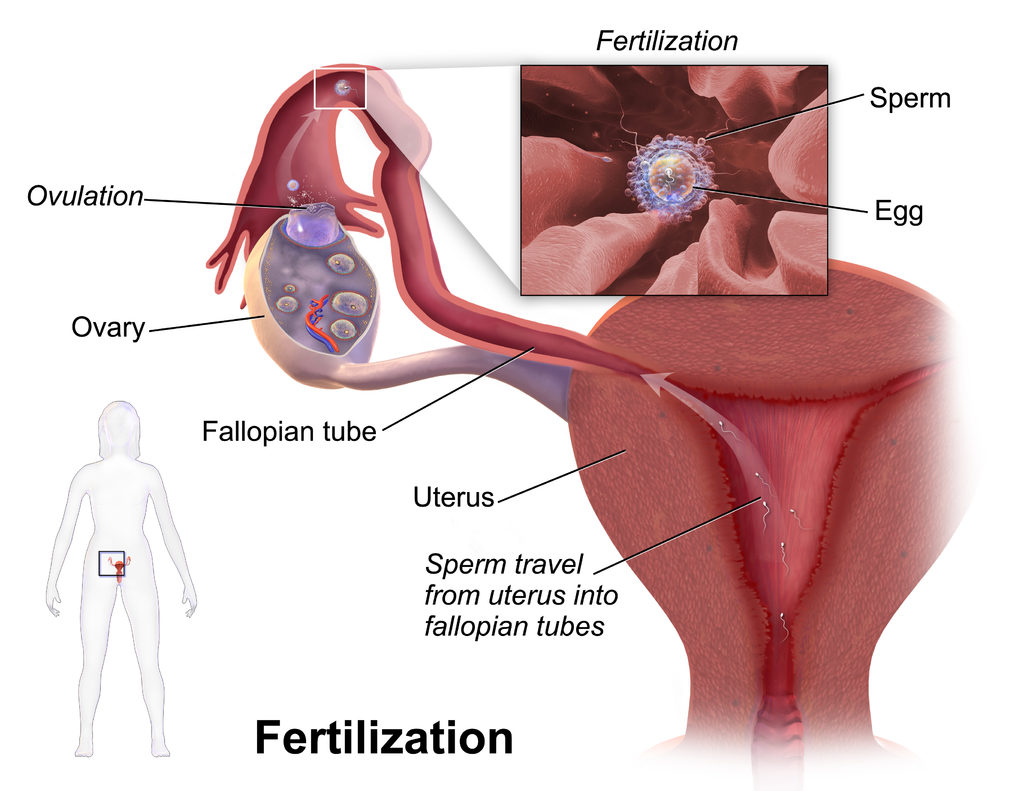
- Fertilization
The fusion of haploid gametes, egg and sperm, to form the diploid zygote.
- Fetus
An unborn offspring of a mammal, in particular an unborn human baby more than eight weeks after conception.
- Fibroblast
A cell in connective tissue which produces collagen and/or other protein fibers.
- Fibrous joint
An immovable joint in which bones are connected by collagen fibers; also called a suture.
- Fight-or-flight response
An involuntary human body response mediated by the nervous and endocrine systems that prepares the body to fight or flee from perceived danger.
- Filtrate
The fluid filtered from blood, called filtrate, passes through the nephron, much of the filtrate and its contents are reabsorbed into the body.
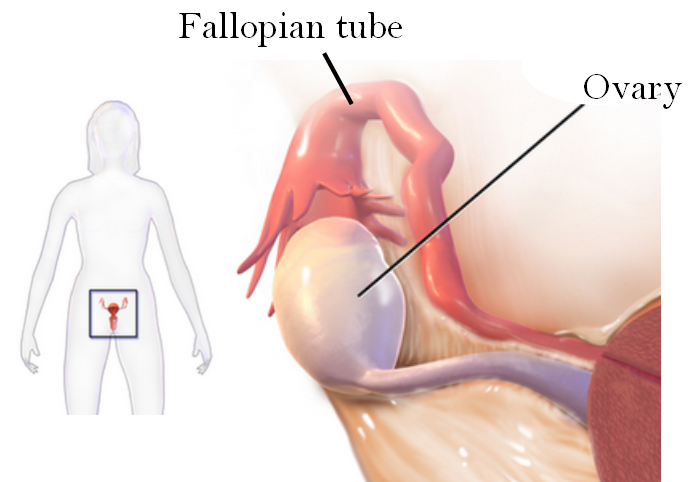
- Fimbriae
Small, fingerlike projections at the end of the oviducts, through which eggs move from the ovaries to the uterus. The fimbriae are connected to the ovary.
- Flagella
A whip-like structure that allows a cell to move.
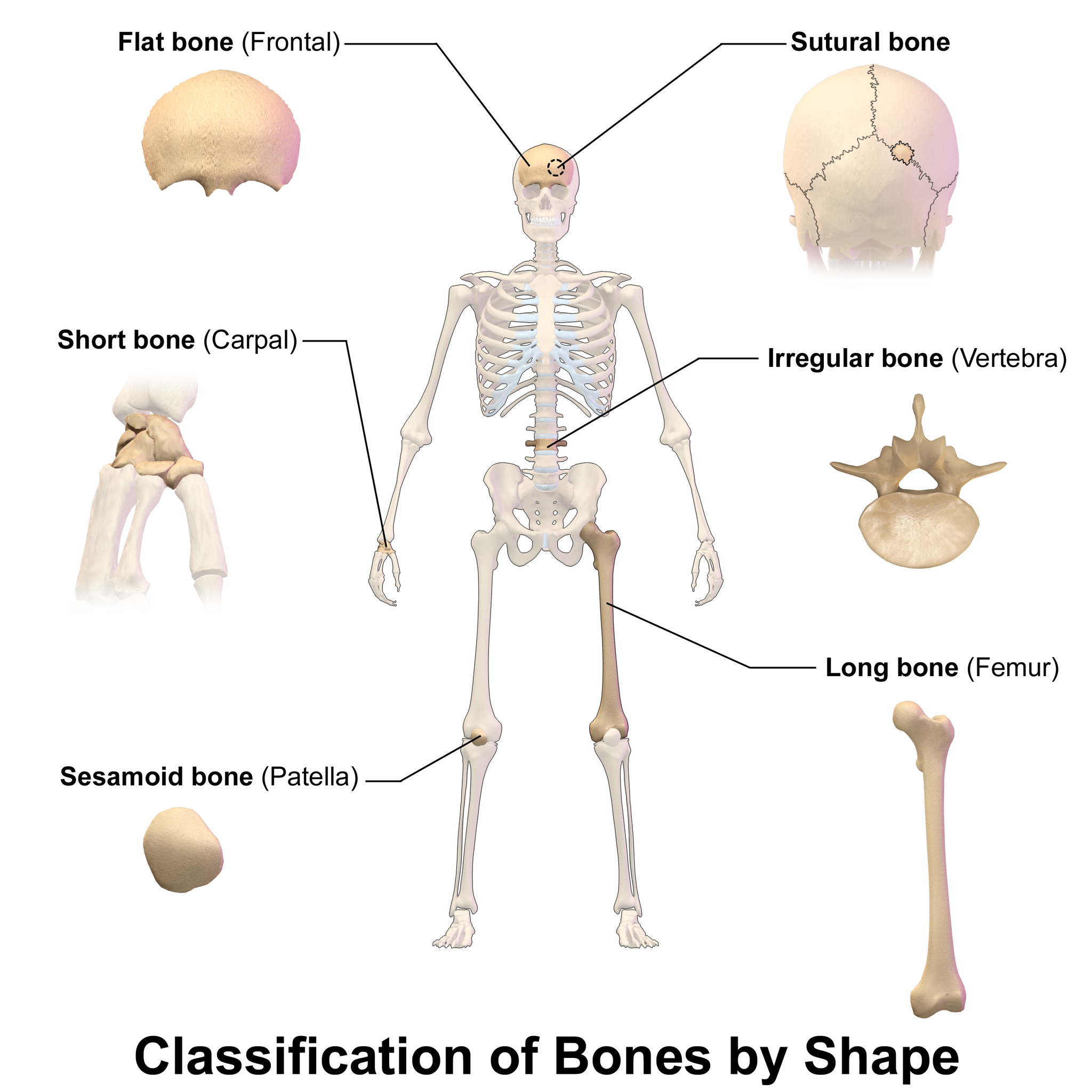
- Flat bones
Bones made up of a layer of spongy bone between two thin layers of compact bone. They have a flat shape, not rounded. Examples include the skull and rib bones. Flat bones have marrow, but they do not have a bone marrow cavity.
- Flexibility exercise
Any physical activity that stretches and lengthens muscles.
- Fluid connective tissue
A form of connective tissue in which the matrix is in a liquid state. Examples include blood and lymph.
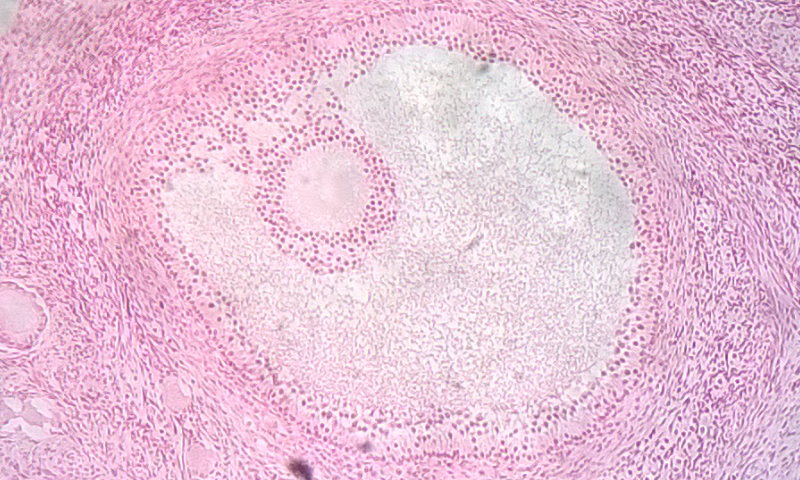
- Follicle
An anatomical structure that consists of a small cluster of cells, surrounding a central cavity.
- Follicle stimulating hormone
A hormone secreted by the anterior pituitary gland which promotes the formation of ova or sperm.
- Follicular phase
The phase of the ovarian cycle during which follicles in the ovary mature. It ends with ovulation. The main hormones controlling this stage are follicle-stimulating hormone and gonadotropin-releasing hormone.
- Food
Any substance consumed to provide nutritional support for an organism.
- Foreskin
The retractable roll of skin covering the end of the penis.
- Founder effect
The loss of genetic variation that occurs when a new population is established by a very small number of individuals from a larger population.
- Frameshift mutation
A genetic mutation caused by a deletion or insertion in a DNA sequence that shifts the way the sequence is read.
- Free margin
The part of the nail that protrudes beyond the end of the finger or toe; the part that is cut or filed to keep the nail trimmed.
- Frenulum
The location where the foreskin meets the underside of the penis. It looks like a small V just below the glans. Usually part of it remains after circumcision.
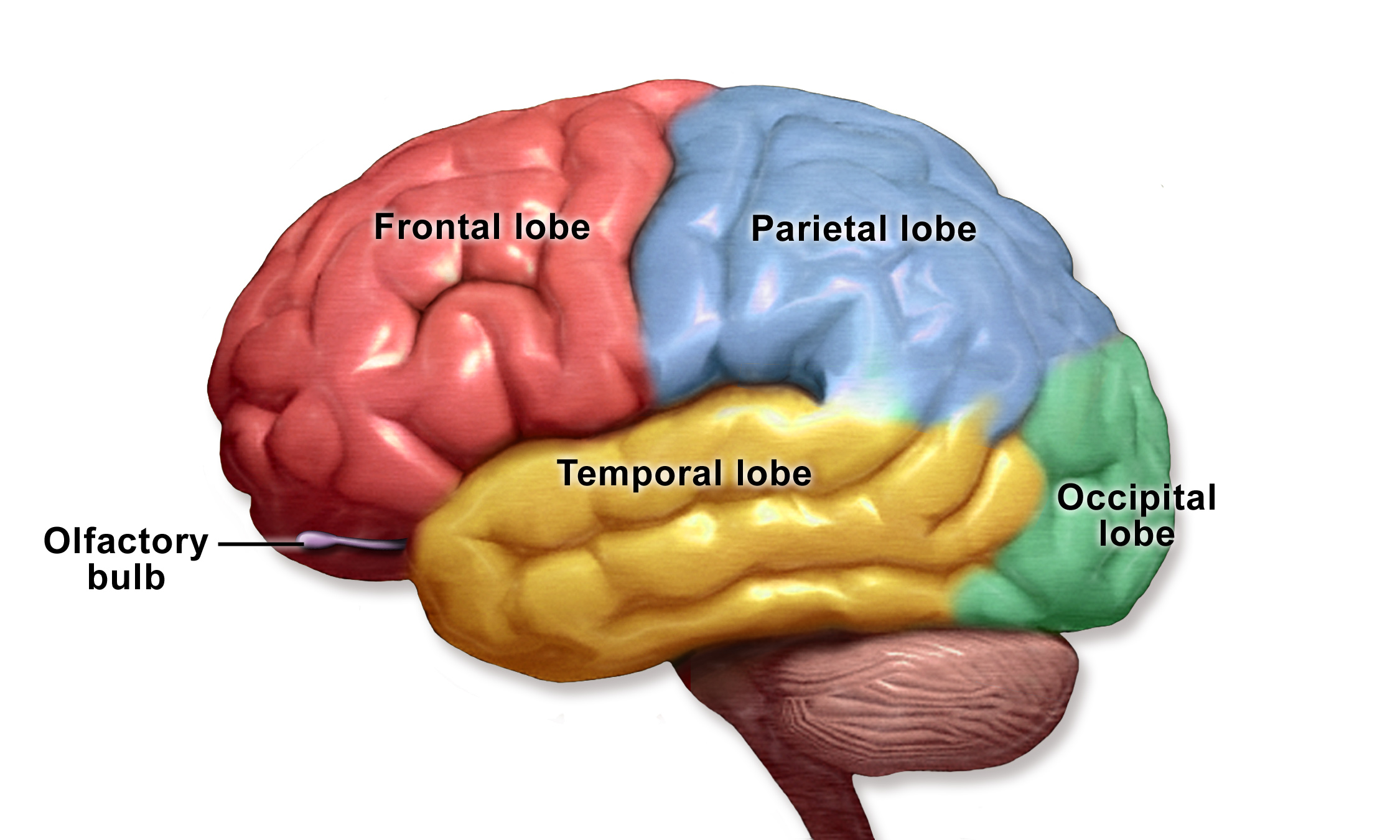
- Frontal lobe
A part of each hemisphere of the cerebrum that controls executive functions such as reasoning and language.
- Frostbite
An injury to body tissues caused by exposure to extreme cold, typically affecting the nose, fingers, or toes and sometimes resulting in gangrene.
- GABA
A naturally occurring amino acid that works as a neurotransmitter in your brain. Neurotransmitters function as chemical messengers. GABA is considered an inhibitory neurotransmitter because it blocks, or inhibits, certain brain signals and decreases activity in your nervous system.
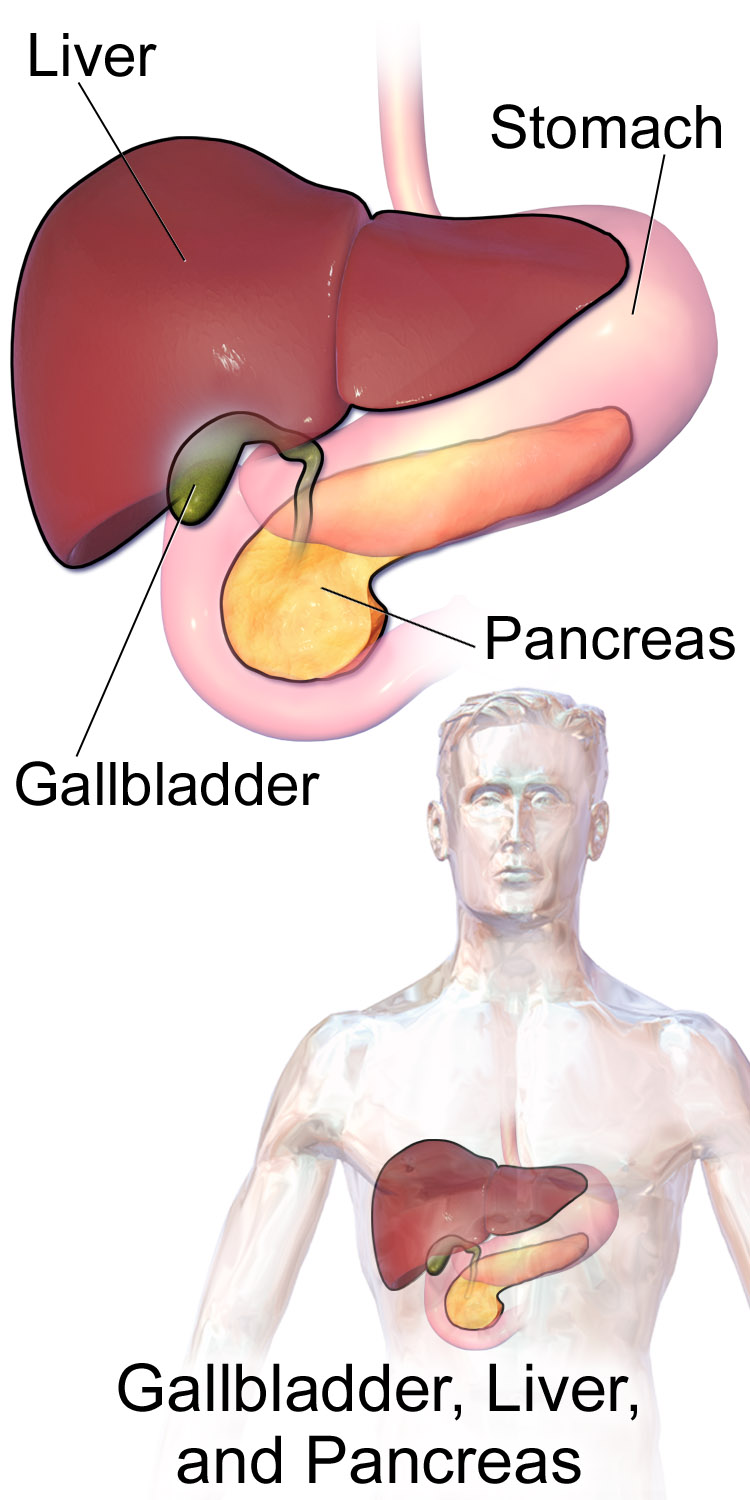
- Gallbladder
A sac-like organ that stores bile from the liver and secretes it into the duodenum of the small intestine as needed for digestion.
- Gamete
A mature haploid male or female germ cell which is able to unite with another of the opposite sex in sexual reproduction to form a zygote.
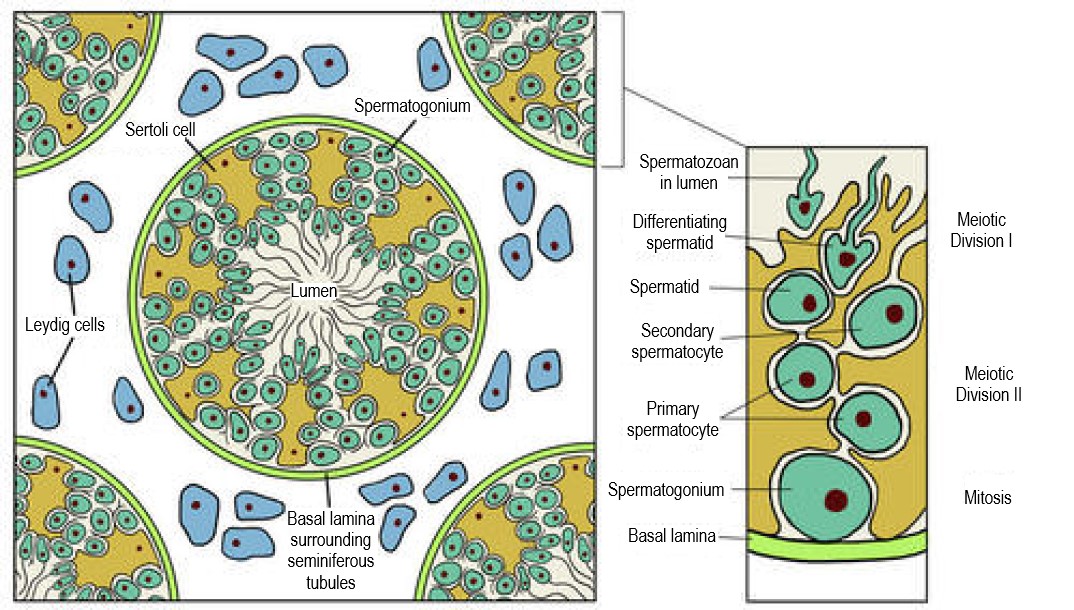
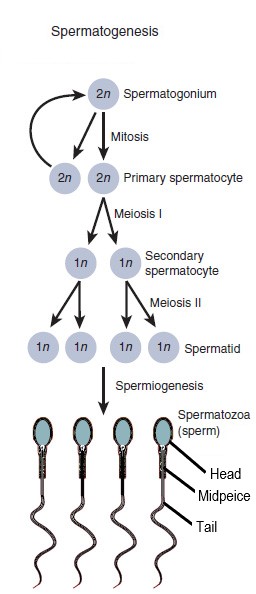
- Gametogenesis
The process whereby a haploid cell (n) is formed from a diploid cell (2n) through meiosis and cell differentiation. Gametogenesis in the male is known as spermatogenesis and produces spermatozoa. Gametogenesis in the female is known as oogenesis and result in the formation of ova.
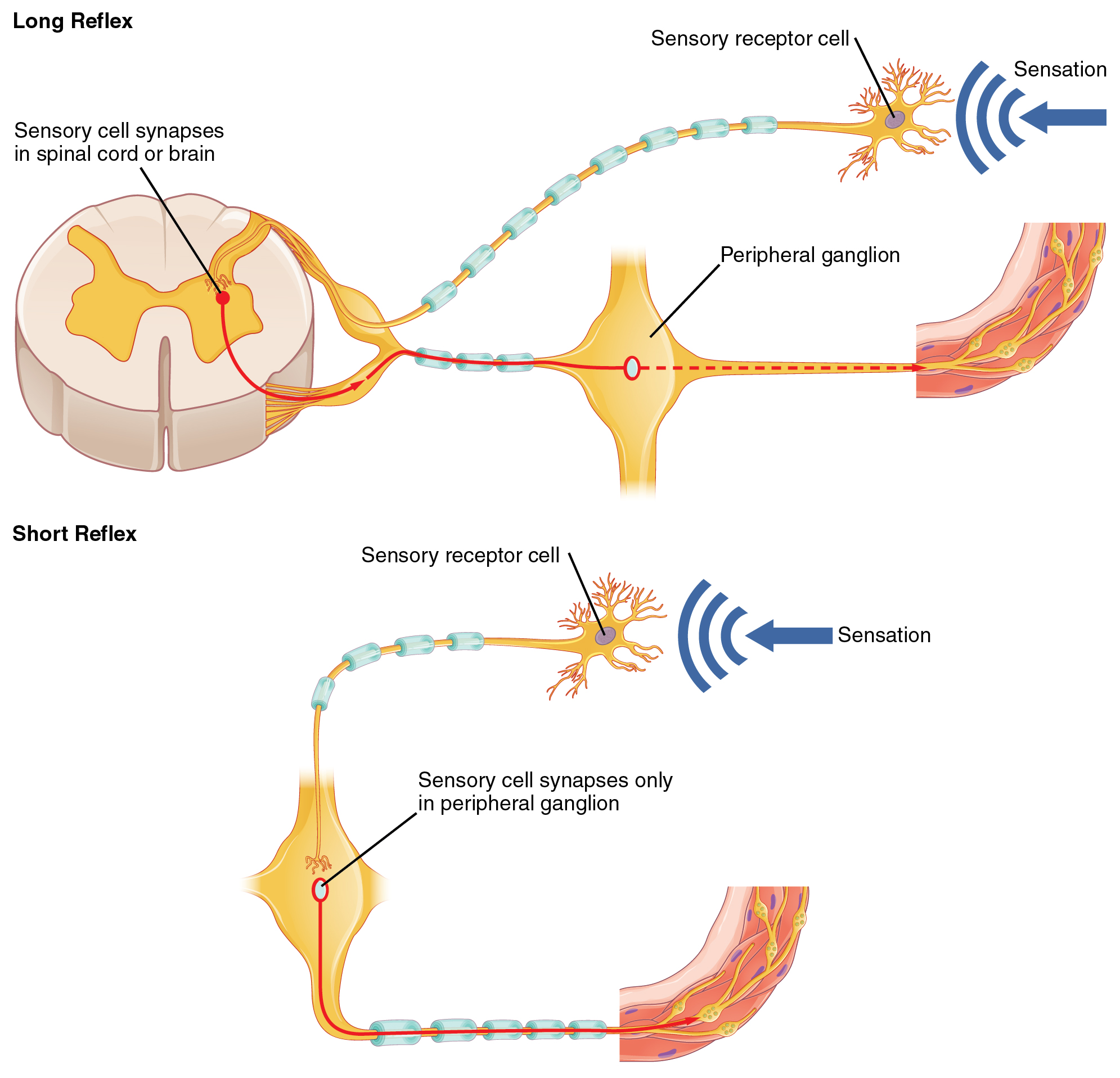
- Ganglia
A structure containing neuronal cell bodies in the peripheral nervous system.
- Ganglion
Structures containing neuronal cell bodies in the peripheral nervous system.
- Gas exchange
Biological process through which gases are transferred across cell membranes to either enter or leave the blood.
- Gastroenteritis
An acute and usually self-limiting infection of the gastrointestinal tract by pathogens; also known as infectious diarrhea.
- Gastrointestinal (GI) tract
The organs of the digestive system through which food passes during digestion, including the mouth, pharynx, esophagus, stomach, and small and large intestines.
- Gene
A sequence of nucleotides in DNA or RNA that codes for a molecule that has a function.
- Gene cloning
The process of isolating and making copies of a gene.
- Gene expression
The process by which information from a gene is used in the synthesis of a functional protein.
- Gene flow
The transfer of genetic variation from one population to another. If the rate of gene flow is high enough, then two populations are considered to have equivalent allele frequencies and therefore effectively be a single population.
- Gene theory
A theory which states that the characteristics of living things are controlled by genes that pass from parents to offspring.
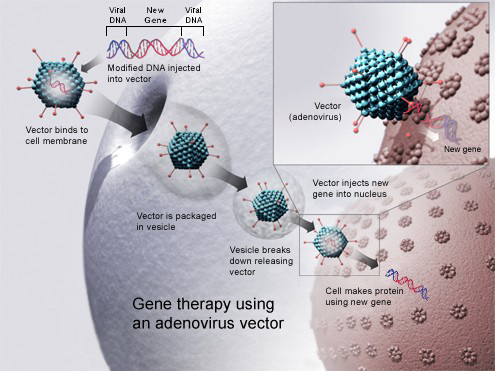
- Gene therapy
An experimental technique that uses genes to treat or prevent disease.
- General senses
A sense which lacks specialized sensory organs and is monitored instead by sensory receptors all over the body, such as the sense of touch.
- Generalist
An organism able to utilize many food sources and therefore able to flourish in many habitats.
- Genetic disorders
Diseases, syndromes, or other abnormal conditions caused by mutations in one or more genes, or by chromosomal alterations.
- Genetic drift
Variation in the relative frequency of different genotypes in a small population, owing to the chance disappearance of particular genes as individuals die or do not reproduce.
- Genetic engineering
The use of technology to change the genetic makeup of living things for human purposes.
- Genetics
A branch of biology concerned with the study of genes, genetic variation, and heredity in organisms.
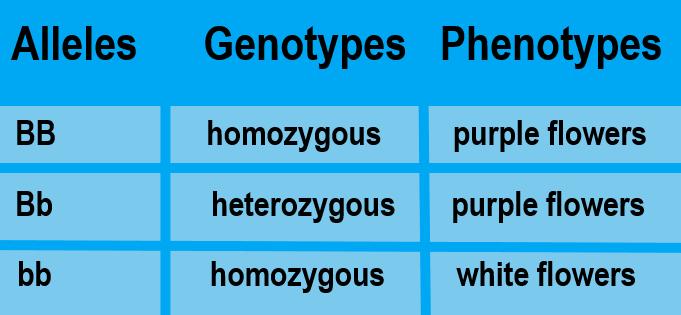
- Genotype
The part of the genetic makeup of a cell, and therefore of any individual, which determines one of its characteristics (phenotype).
- Germ theory of disease
Microorganisms, known as pathogens, can lead to disease.
- Germline mutation
Mutation in cells destined to become egg or sperm cells. These mutations can be passed to offspring.
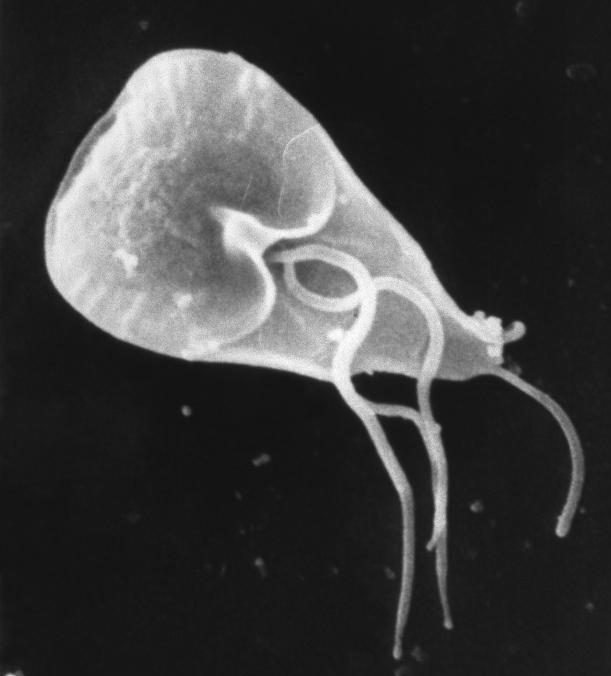
- Giardiasis
A type of gastroenteritis caused by a single-celled protozoan parasite named Giardia lamblia that typically spreads through contaminated food or water via a fecal-oral route.
- Gland
A group of cells in an animal's body that synthesizes substances (such as hormones) for release into the bloodstream (endocrine gland) or into cavities inside the body or its outer surface (exocrine gland).
- Glans penis
The rounded head (or tip) of the penis.
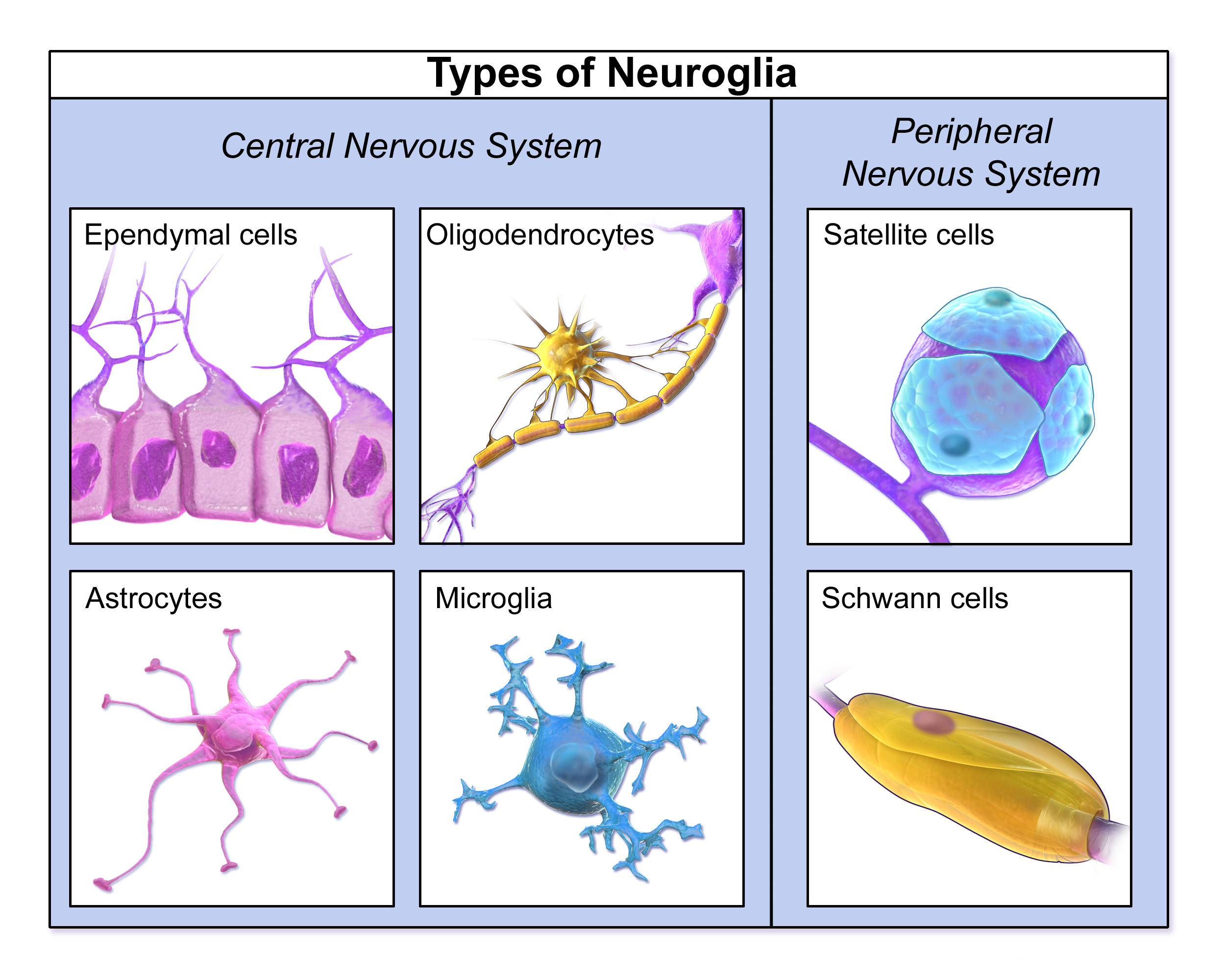
- Glial cell (neuroglia)
A nervous system cell that provides support for neurons and helps them transmit nerve impulses.
- Glomerular capsule
A structure surrounding the glomerulus of a nephron in a kidney, also known as the Bowman's capsule, into which substances that are filtered out of blood are passed to the renal tubule.
- Glomerulus (plural glomeruli)
A network of capillaries in the nephron of a kidney where substances are filtered out of the blood.
- Glucagon
A peptide hormone, produced by alpha cells of the pancreas. It works to raise the concentration of glucose and fatty acids in the bloodstream, and is considered to be the main catabolic hormone of the body. It is also used as a medication to treat a number of health conditions.
- Glucose
Glucose (also called dextrose) is a simple sugar with the molecular formula C6H12O6. Glucose is the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, using energy from sunlight.
- Glutamate
A chemical that nerve cells use to send signals to other cells. It is by a wide margin the most abundant excitatory neurotransmitter in the vertebrate nervous system.
- Gluten
A substance present in cereal grains, especially wheat, that is responsible for the elastic texture of dough. A mixture of two proteins, it causes illness in people with celiac disease.
- Glycogen
A multi-branched polysaccharide of glucose that serves as a form of energy storage in animals, fungi, and bacteria.
- Glycolysis
The metabolic pathway that converts glucose C₆H₁₂O₆, into pyruvate. The free energy released in this process is used to form the high-energy molecules ATP and NADH. Glycolysis is a sequence of ten enzyme-catalyzed reactions.
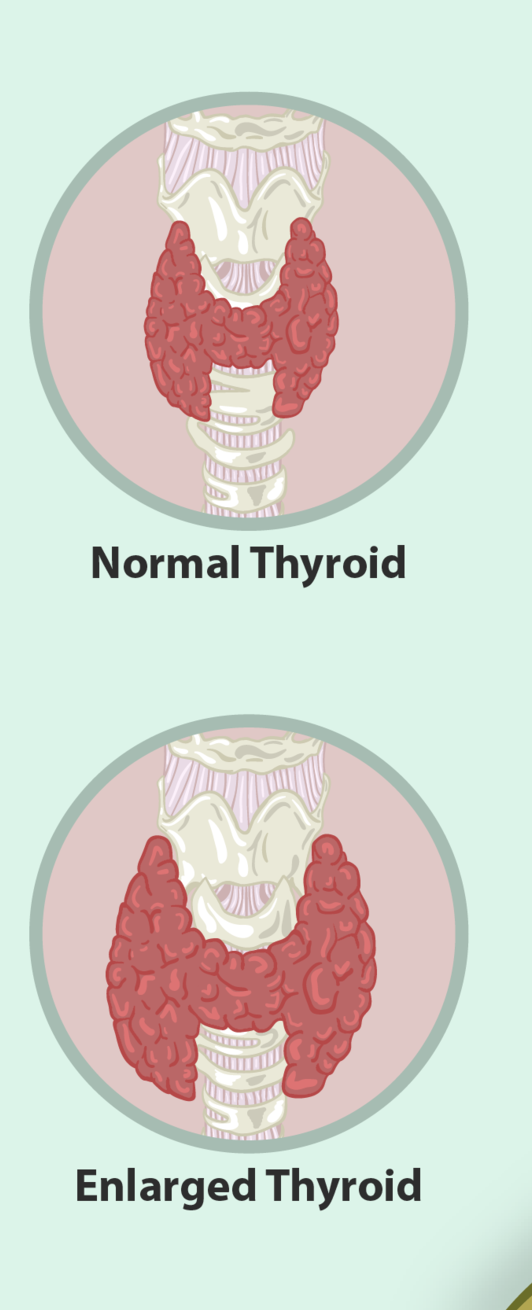
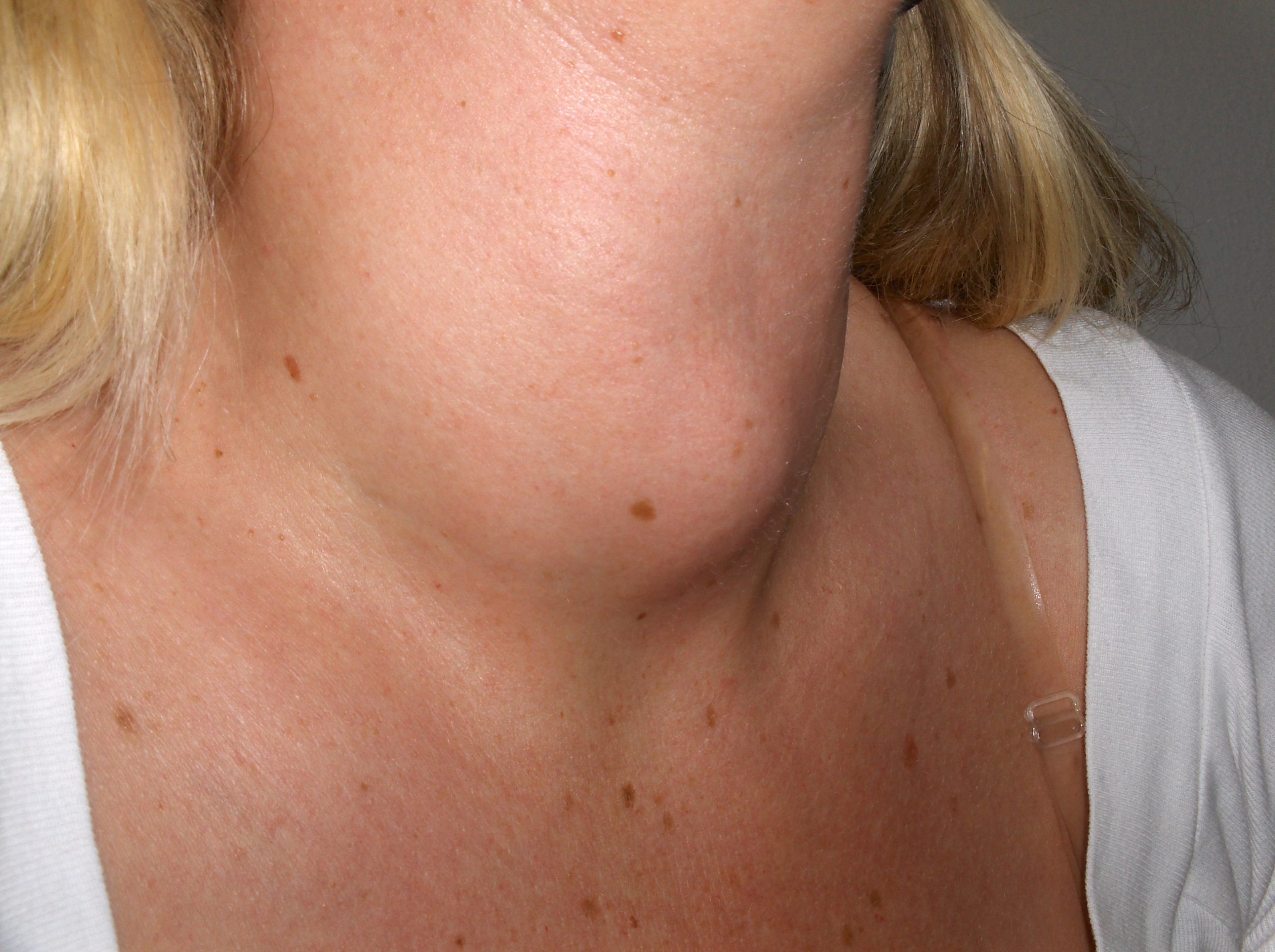
- Goiter
An abnormal enlargement of the thyroid gland.
- Golgi apparatus
A membrane-bound organelle found in eukaryotic cells made up of a series of flattened stacked pouches with the purpose of collecting and dispatching protein and lipid products received from the endoplasmic reticulum (ER). Also referred to as the Golgi complex or the Golgi body.
- Gonad
One of a pair of organs that secrete sex hormones and produce gametes; testis in males and ovary in females.
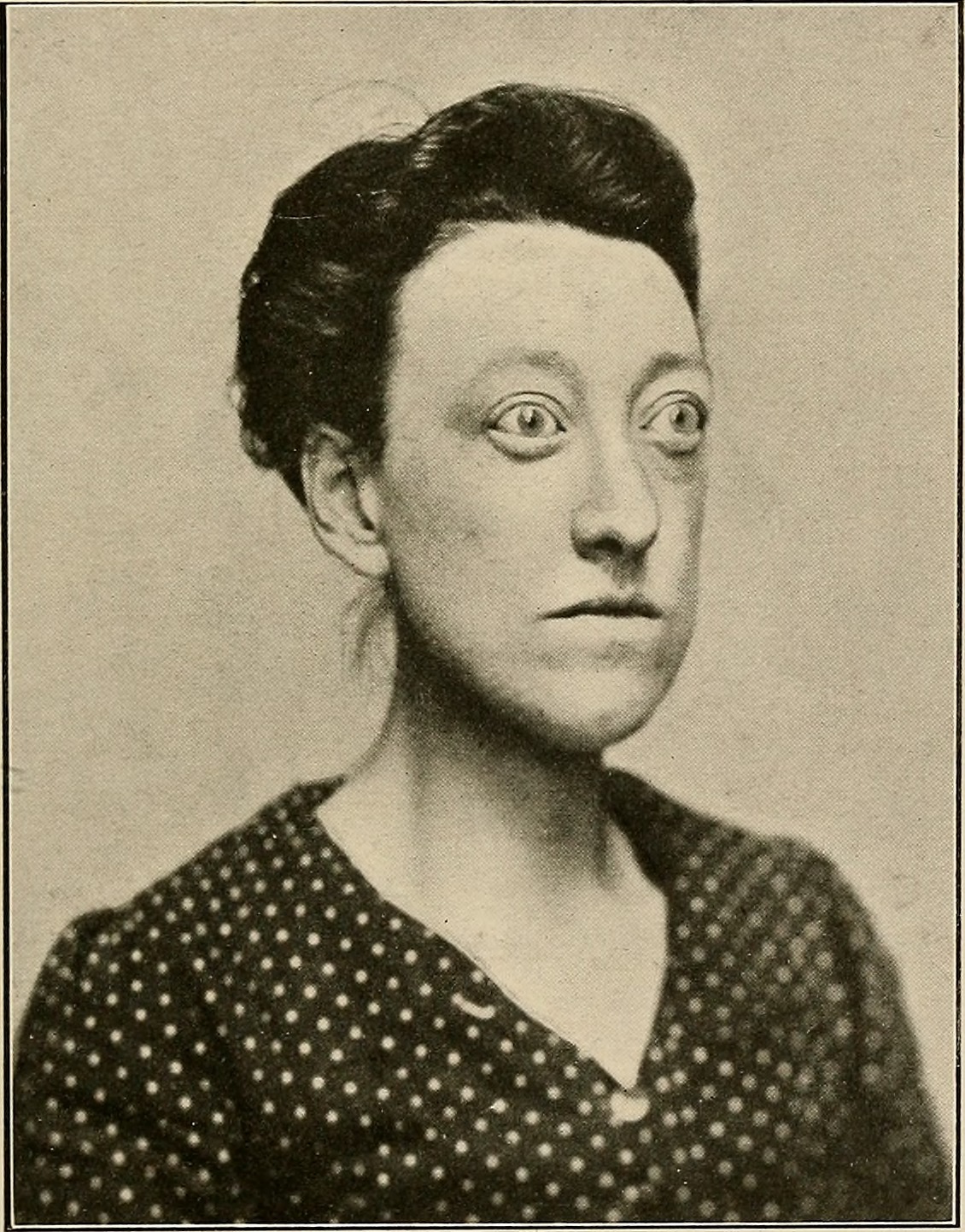
- Graves’ disease
An autoimmune disorder in which abnormal antibodies produced by the immune system stimulate the thyroid gland to secrete excessive quantities of its hormones.
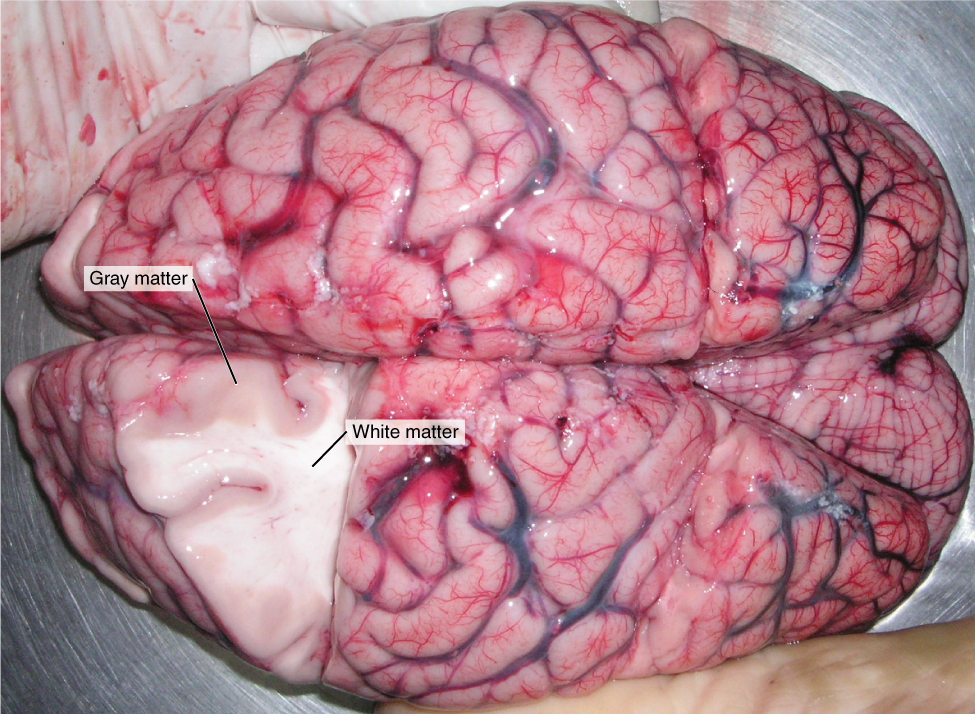
- Gray matter
A type of nervous tissue that is found only in the brain and spinal cord and consists mainly of un-myelinated cell bodies and dendrites of neurons.
- Growth hormone
A hormone secreted by the anterior pituitary gland that stimulates growth in cells all over the body.
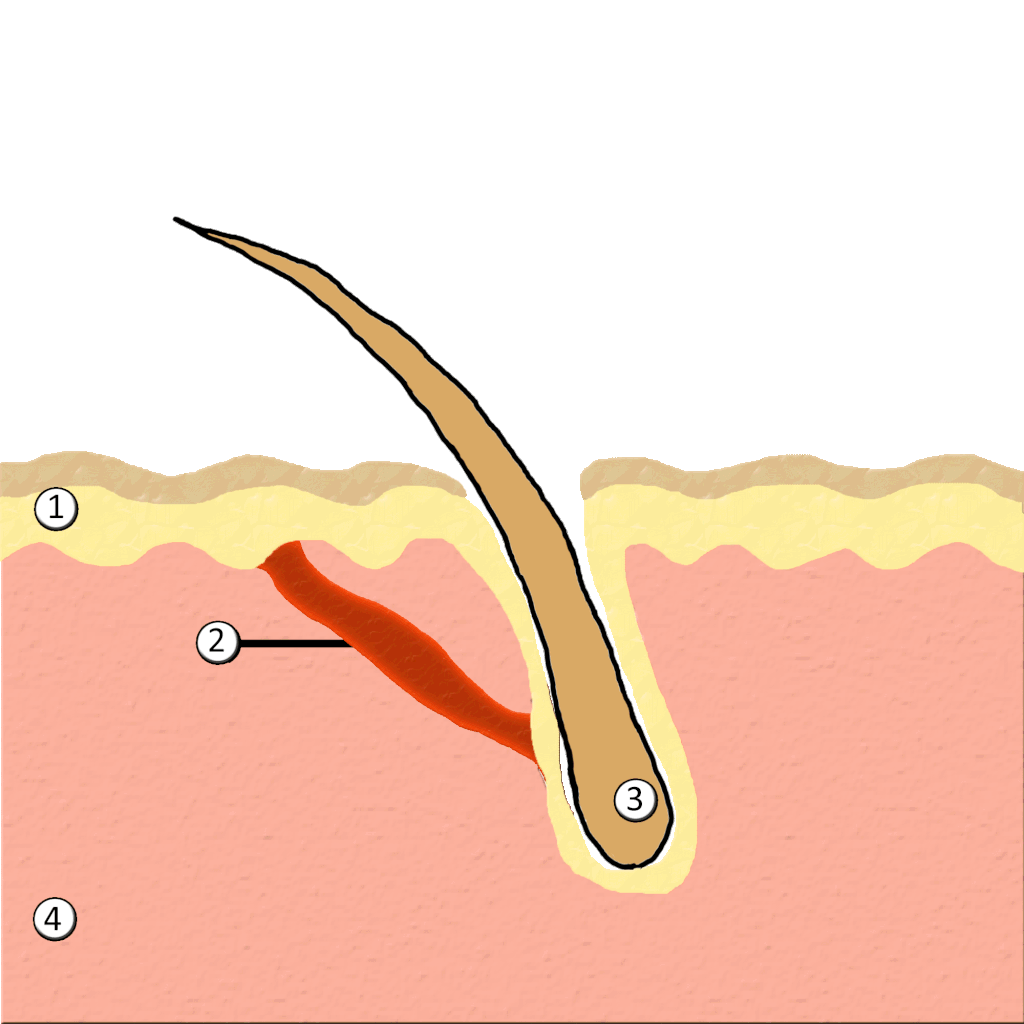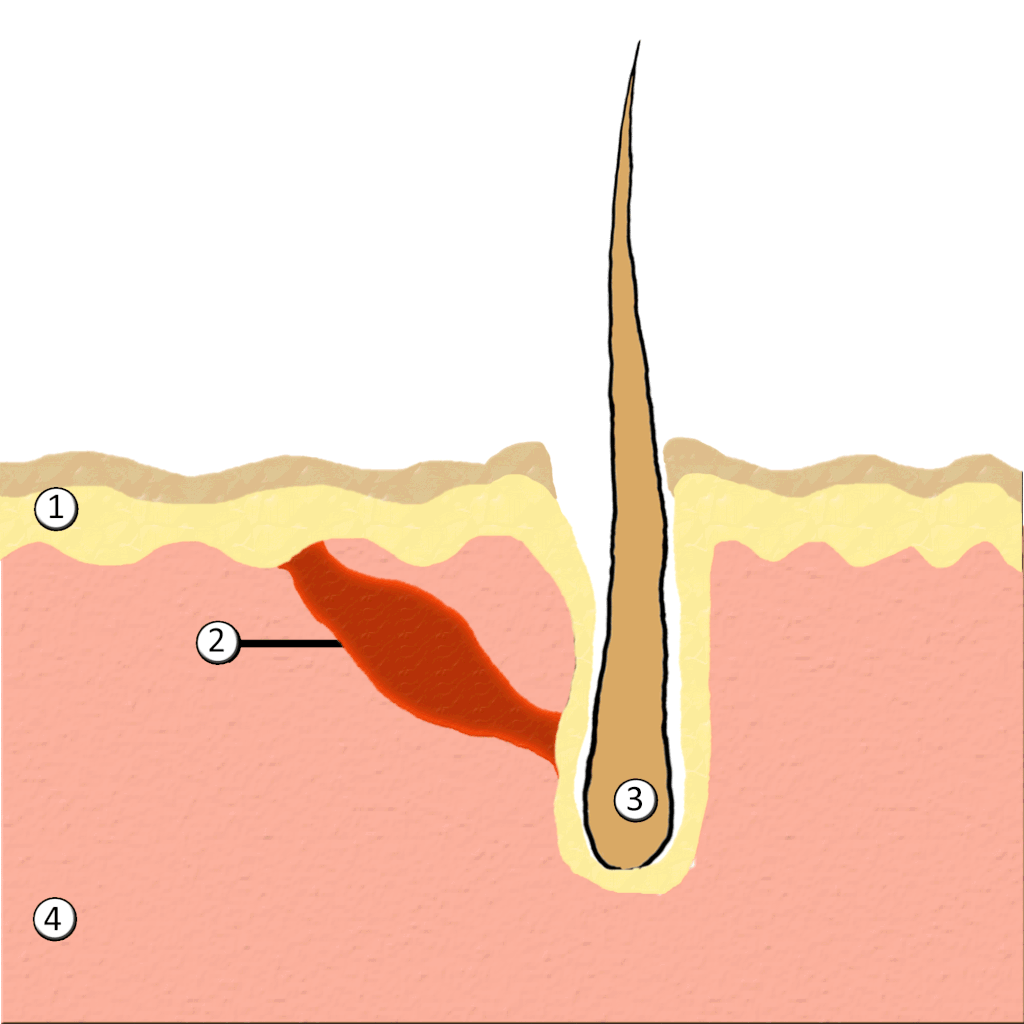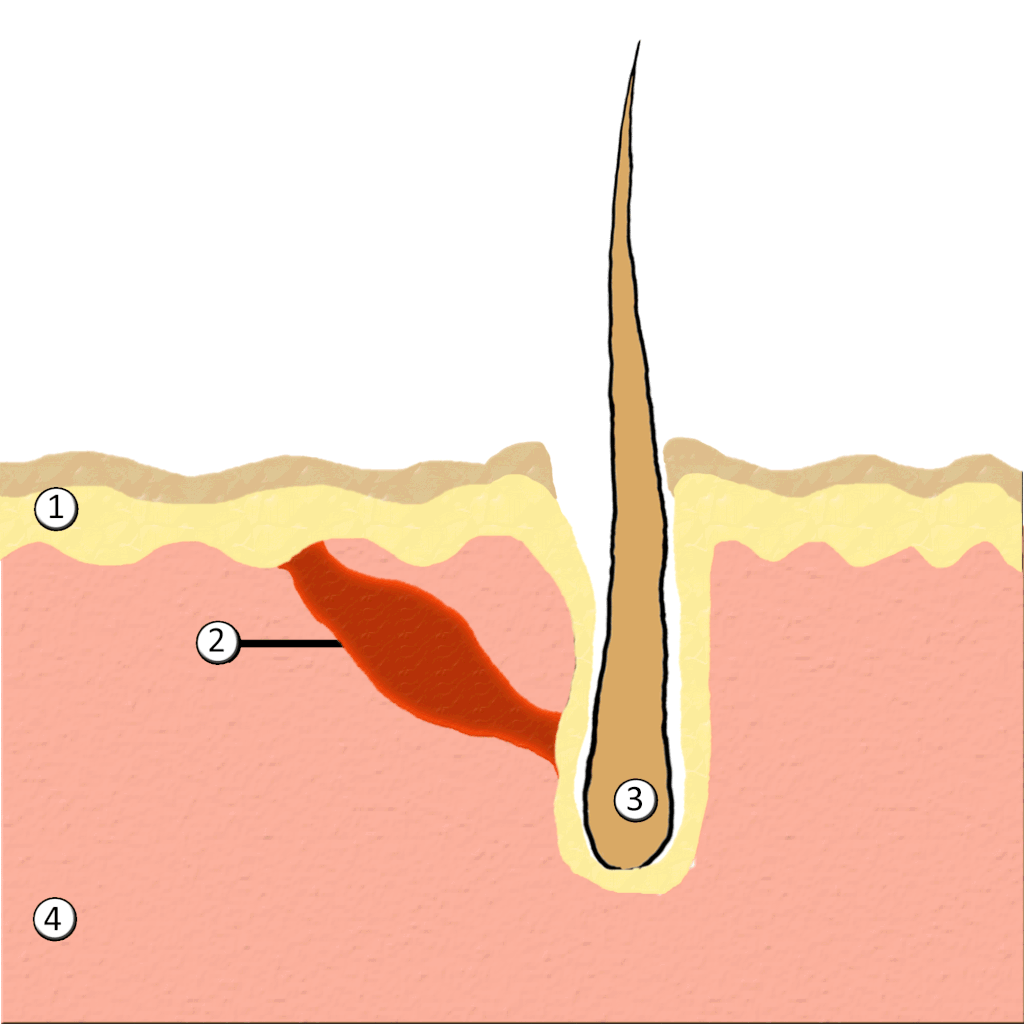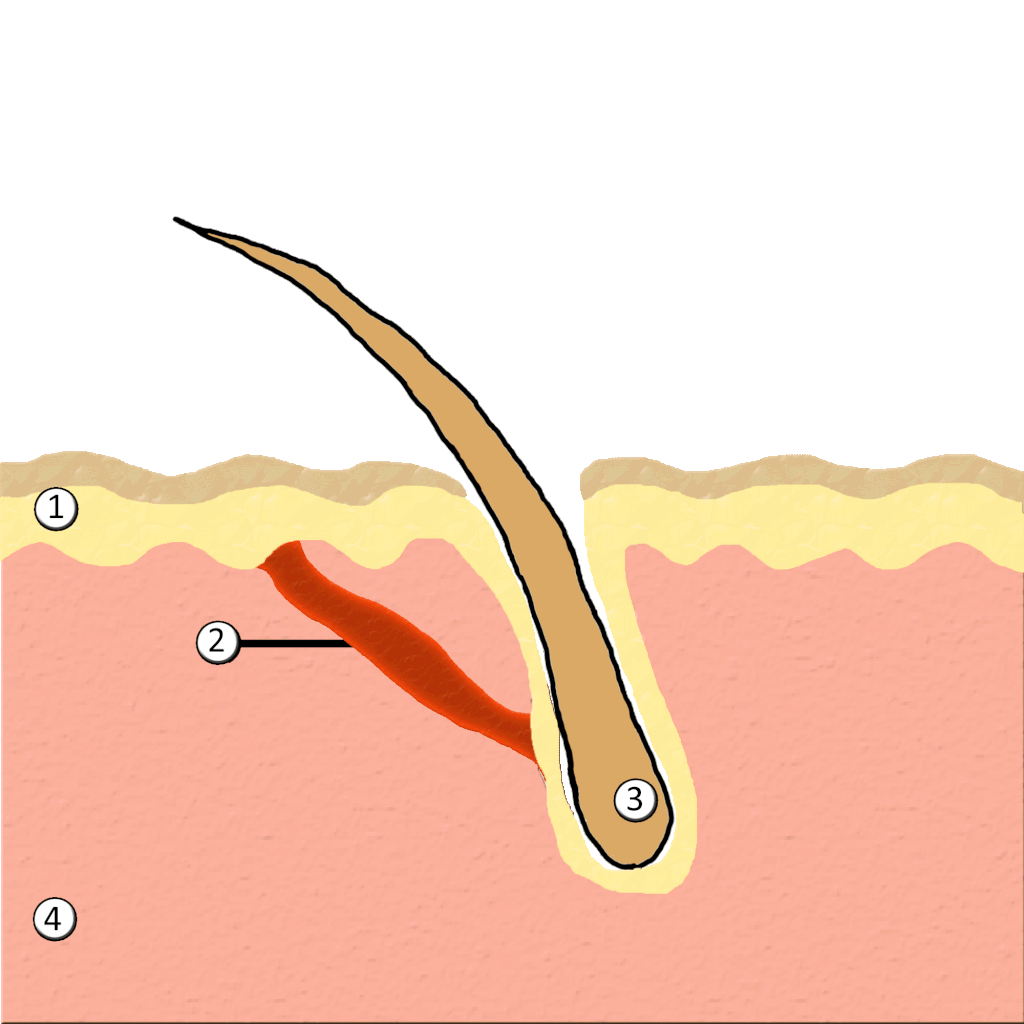
- Hair
A filament made of tightly packed, keratin-filled keratinocytes that grows out of a hair follicle in the dermis of the skin.
- Hair cortex
Located between the hair cuticle and medulla and is the thickest hair layer. It also contains most of the hair's pigment, giving the hair its color. The pigment in the cortex is melanin, which is also found in skin.
- Hair cuticle
The outermost part of the hair shaft. It is formed from dead cells, overlapping in layers, which form scales that strengthen and protect the hair shaft.
- Hair follicle
A structure in the dermis of skin where a hair originates.
- Hair medulla
The innermost layer of the hair shaft. This nearly invisible layer is the most soft and fragile, and serves as the pith or marrow of the hair.
- Hair root
The part of a hair that is located within the hair follicle and consists of living keratinocytes.
- Hair shaft
A part of a hair that is visible above the surface of the skin and consists of dead keratinocytes.
- Hallucinogens
A type of psychoactive drug that causes hallucinations and other perceptual anomalies, as well as subjective changes in thoughts, emotions, and consciousness.
- Haploid
The term used when a cell has half the usual number of chromosomes.
- Head of sperm
The part of the sperm that contains the nucleus.
- Hearing
The ability to sense sound waves.
- Heart
A muscular organ in the chest that pumps blood through blood vessels when it contracts.
- Heart attack
The blockage of blood flow to heart muscle tissues that may result in the death of cardiac muscle fibers.
- Heart failure
A term used to describe a heart that cannot keep up with its workload. The body may not get the oxygen it needs. Heart failure is a serious condition, and usually there's no cure.
- HeLa cells
An immortal cell line used in scientific research. It is the oldest and most commonly used human cell line. The line was derived from cervical cancer cells taken on February 8, 1951 from Henrietta Lacks, a patient who died of cancer on October 4, 1951. The cell line was found to be remarkably durable and prolific, which warrants its extensive use in scientific research.
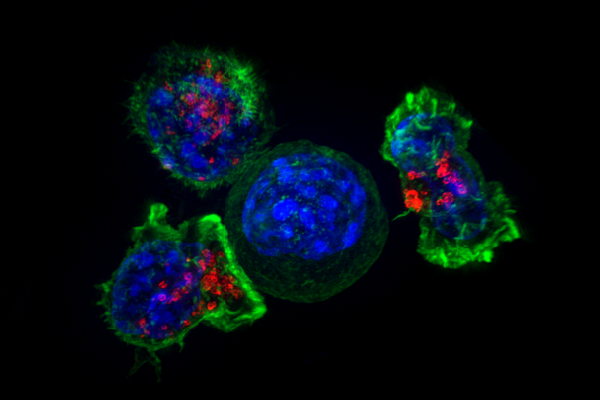
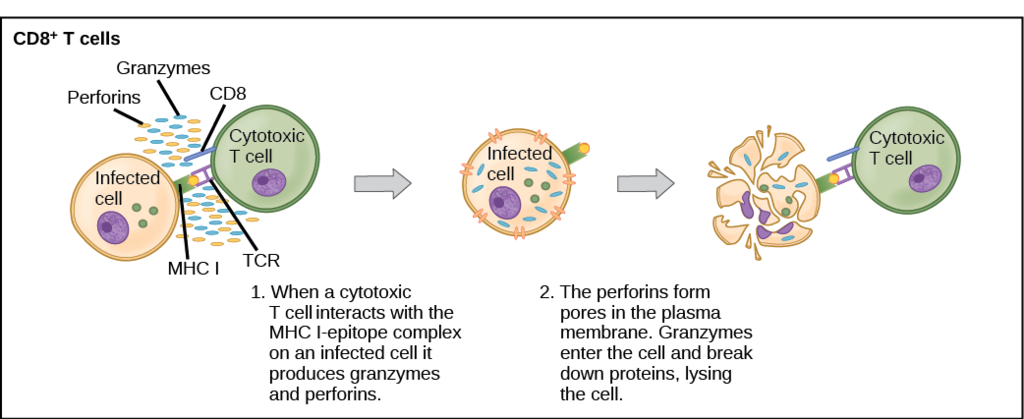
- Helper T cell
A type of immune cell that stimulates killer T cells, macrophages, and B cells to make immune responses. A helper T cell is a type of white blood cell and a type of lymphocyte.
- Hematopoiesis
The process in which red blood cells, white blood cells, and platelets are produced by red bone marrow.
- Hemisphere (of the brain)
One of two halves (left and right) of the cerebrum of the human brain.
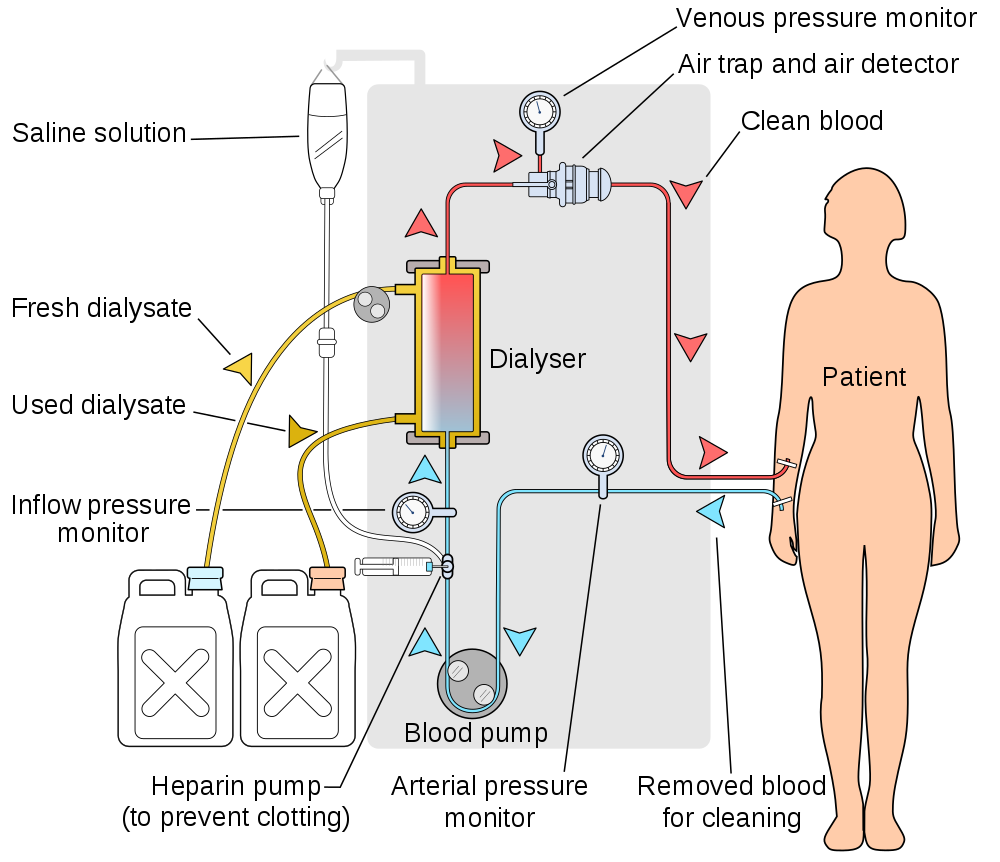
- Hemodialysis
A medical procedure for patients with kidney failure in which wastes and excess water are artificially filtered out of blood by passing it through a machine.
- Hemoglobin
An oxygen-binding protein containing iron that is the principal component of red blood cells.
- Hemophilia
Any of several genetic disorders that cause dysfunction in the blood-clotting process, leading to uncontrolled bleeding from even minor injuries.
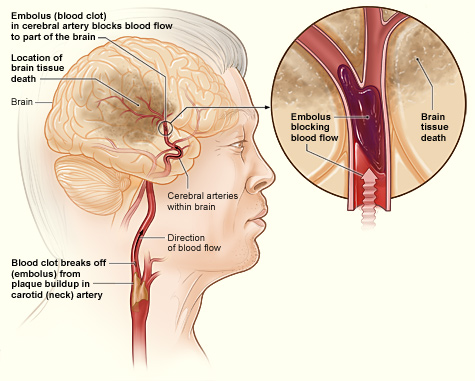
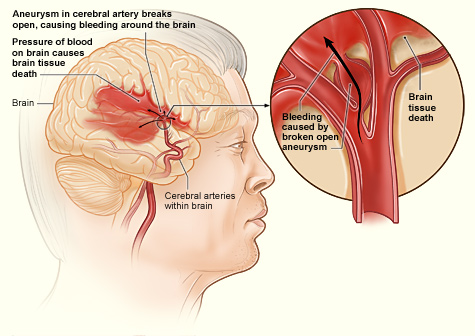
- Hemorrhagic stroke
An event which occurs when a weakened blood vessel ruptures. Two types of weakened blood vessels usually cause hemorrhagic stroke: aneurysms and arteriovenous malformations (AVMs).
- Hemostasis
A process to prevent and stop bleeding, meaning to keep blood within a damaged blood vessel (the opposite of hemostasis is hemorrhage). It is the first stage of wound healing. This involves coagulation, blood changing from a liquid to a gel.
- Heparin
A compound occurring in the liver and other tissues which inhibits blood coagulation.
- Hepatocyte
A liver cell.
- Heterotroph
An organism that cannot produce its own food, relying instead on the intake of nutrition from other sources of organic carbon, mainly plant or animal matter. In the food chain, heterotrophs are primary, secondary and tertiary consumers, but not producers.
- Heterozygote
An individual who has two different forms of a particular gene, one inherited from each parent.
- High altitude sickness
The negative health effect of high altitude, the mildest form being acute mountain sickness (AMS), caused by rapid exposure to low amounts of oxygen at high elevation. Symptoms may include headaches, vomiting, tiredness, trouble sleeping, and dizziness.
- Hilum (of the kidney)
The entry and exit site for structures servicing the kidneys: vessels, nerves, lymphatics, and ureters.
- Hindbrain
One of the three major regions of the human brain, located at the lower back part of the brain. It includes most of the brainstem and a dense coral-shaped structure called the cerebellum. The brainstem is one of the most important parts of the entire central nervous system, because it connects the brain to the spinal cord and coordinates many vital functions, such as breathing and heartbeat.
- Hinge joint
A synovial bone joint in which the articular surfaces are molded to each other in such a manner as to permit motion only in one plane.
- Hippocampus
A complex brain structure embedded deep into temporal lobe. It has a major role in learning and memory.
- Histamine
A compound which is released by cells in response to injury and in allergic and inflammatory reactions, causing contraction of smooth muscle and dilation of capillaries.
- Histology
The study of the microscopic anatomy and cells and tissues.
- HIV (human immunodeficiency viruses)
Either of two species of Lentivirus that infect humans. Over time, they cause acquired immunodeficiency syndrome, a condition in which progressive failure of the immune system allows life-threatening opportunistic infections and cancers to thrive.
- Hoax
An intentional deception for the purpose of humour or malice.
- Homeobox genes
A large group of similar genes that direct the formation of many body structures during early embryonic development.
- Homeodomain
The part of a protein that attaches (binds) to specific regulatory regions of the target genes.
- Homeostasis
The ability of an organism to maintain constant internal conditions despite external changes.
- Homeostatic imbalance
A condition in which cells may not get everything they need or toxic wastes may accumulate because of the failure of a homeostatic mechanism.
- Hominid
Of, relating to, or being a member of a family (Hominidae) of erect, bipedal, primate mammals that includes recent humans together with extinct ancestral and related forms and in some recent classifications the gorilla, chimpanzee, and orangutan.
- Homologous chromosomes
Two pieces of DNA within a diploid organism which carry the same types genes, one from each parental source.
- Homologous structure
Structures that are similar in related species because it was inherited from a common ancestor; or structure that develops from the same undifferentiated embryonic tissue in males and females of the same species, such as the testis and ovary in humans.
- Homozygote
An organism with identical pairs of genes (or alleles) for a specific trait. If both of the two gametes (sex cells) that fuse during fertilization carry the same form of the gene for a specific trait, the organism is said to be homozygous for that trait.
- Hormonal contraception
A method of birth control which makes use of hormones such as estrogen and/or progesterone to prevent pregnancy by interfering with ovulation.
- Hormones
A hormone is a signaling molecule produced by glands in multicellular organisms that target distant organs to regulate physiology and behavior.
- Human biology
An interdisciplinary area of study that examines humans through the influences and interplay of many diverse fields including genetics, evolution, physiology, anatomy, nutrition, ecology, etc.
- Human chorionic (hCG)
Human chorionic gonadotropin is a hormone produced by cells that are surrounding a growing embryo, which eventually forms the placenta after implantation. The presence of hCG is detected in some pregnancy tests.
- Human genome
Refers to all the DNA of the human species.
- Human Genome Project
An international scientific research project with the goal of determining the base pairs that make up human DNA, and of identifying and mapping all of the genes of the human genome from both a physical and a functional standpoint.
- Humanpapillomavirus (HPV)
A sexually transmitted virus that may cause genital warts and cervical cancer.
- Hunting response (Lewis reaction)
A process of alternating vasoconstriction and vasodilation in extremities exposed to cold. The term Lewis reaction is used too, named after Thomas Lewis, who first described the effect in 1930.
- Hybrids
The offspring resulting from combining the qualities of two organisms of different breeds, varieties, species or genera through sexual reproduction.
- Hydrocephalus
A condition where there is an abnormal build up of CSF (cerebrospinal fluid) in the cavities (ventricles) of the brain, also called water in the brain. The build-up is often caused by an obstruction that prevents proper fluid drainage.
- Hydrogen bond
A hydrogen bond is the attractive force between the hydrogen attached to an electronegative atom of one molecule and an electronegative atom of a different molecule.
- Hydronium ion
H+. A positively charged hyrogen atom, which has lost its single electron.
- Hydrophilic
Attracted to water.
- Hydrophobic
Repelled by water.
- Hyperopia
A vision problem in which close objects are out of focus but distant vision is unaffected; also called farsightedness.
- Hypersecretion
A secretion of more than the normal amount of a substance, such as secretion of too much hormone by an endocrine gland.
- Hypertension
A persistently high blood pressure, generally defined as 140/90 mm Hg or higher.
- Hyperthermia
A group of heat-related conditions characterized by an abnormally high body temperature — in other words, the opposite of hypothermia. The condition occurs when the body's heat-regulation system becomes overwhelmed by outside factors, causing a person's internal temperature to rise.
- Hyperthyroidism
A disorder in which the thyroid gland produces excessive amounts of hormones.
- Hypertrophy
An increase in the size of a structure, such as an increase in the size of a muscle through exercise.
- Hyperventilation
Breathing more quickly and shallowly than normal.
- Hyponatremia
A low sodium concentration in the blood often caused by over consumption of water. Mild symptoms include a decreased ability to think, headaches, nausea, and poor balance.
- Hyposecretion
The secretion of less than the normal amount of a substance, such as secretion of too much hormone by an endocrine gland.
- Hypothalamus
A part of the brain that secretes hormones and connects the brain with the endocrine system.
- Hypothermia
A medical emergency that occurs when your body loses heat faster than it can produce heat, causing a dangerously low body temperature. Normal body temperature is around 98.6 F (37 C). Hypothermia occurs as your body temperature falls below 95 F (35 C).
- Hypothesis
A testable proposed explanation for a phenomenon.
- Hypothyroidism
A disorder in which the thyroid gland produces inadequate amounts of hormones.
- Hypoxia
A condition in which the body or a region of the body is deprived of adequate oxygen supply at the tissue level.
- Hysterectomy
An operation to remove a woman's uterus.
- Ileum
The third portion of the small intestine, between the jejunum and the cecum.The ileum helps to further digest food coming from the stomach and other parts of the small intestine.
- Immovable joint
An articulation between bones in which no movement occurs. It is also referred to as synarthrotic (meaning immovable).
- Immune surveillance
A function of the immune system in which it identifies and eliminates tumor cells.
- Immune system
The body system in humans and other animals that protects the organism by distinguishing foreign tissue and neutralizing potentially pathogenic organisms or substances.
- Immunity
The state of being immune from or insusceptible to a particular disease or the like. the condition that permits either natural or acquired resistance to disease. the ability of a cell to react immunologically in the presence of an antigen.
- Immunization
The deliberate exposure of a person to a pathogen in order to provoke an immune response and the formation of memory cells specific to that pathogen.
- Immunodeficiency
The inability of the immune system to fight off pathogens that a normal, healthy immune system would be able to resist because the immune system is damaged.
- Immunotherapy
A treatment for an allergy in which a patient is gradually desensitized to an allergen through periodic injections with increasing amounts of the allergen; or treatment for cancer that attempts to stimulate the immune system to destroy cancer cells.
- Incisor
One of eight (four upper and four lower) blade-like teeth at the front of the mouth that are used to slice off pieces of food.
- Incomplete dominance
A heredity pattern in which phenotype of the heterozygous genotype is distinct from and often intermediate to the phenotypes of the homozygous genotypes.
- Independent alignment
The way in which different genes independently separate from one another when reproductive cells develop. During meiosis, the pairs of homologous chromosome are divided in half to form haploid cells, and this separation, or assortment, of homologous chromosomes is random.
- Infertility
The failure to achieve a successful pregnancy after at least one year of regular, unprotected sexual intercourse.
- Inflammation
The response of the innate immune system that establishes a physical barrier against the spread of infection and repairs tissue damage while causing redness, swelling, and warmth.
- Inflammatory bowel disease
A type of disease in which the immune system attacks the intestines, causing diarrhea and abdominal pain; for example, Crohn’s disease or ulcerative colitis.
- Infundibula
The the wide distal (outermost) portion of each oviduct which catches and channels the ova released by the ovaries.
- Inhalation
Inhalation happens when air or other gases enter the lungs. The action of inhaling or breathing in.
- Inhibin
A gonadal hormone which inhibits the secretion of follicle-stimulating hormone, under consideration as a potential male contraceptive.
- Inhibitory neurotransmitter
A neurotransmitter that decreases the likelihood that a neuron will fire an action potential.
- Innate immune system
A subset of the immune system that makes generic attacks such as inflammation against invading pathogens.
- Insomnia
A sleep disorder in which one has trouble falling and/or staying asleep.
- Insulin
A hormone made by the pancreas that allows your body to use sugar (glucose) from carbohydrates in the food that you eat for energy or to store glucose for future use.
- Integumentary system
The body system comprised of skin and its appendages acting to protect the body from various kinds of damage, such as loss of water or damages from outside.
- Intercalated discs
Unique structural formations found between the myocardial cells of the heart. They play vital roles in bonding cardiac muscle cells together and in transmitting signals between cells.
- Intermembrane space
The space occurring between two or more membranes. In cell biology, it's most commonly described as the region between the inner membrane and the outer membrane of a mitochondrion or a chloroplast.
- Interneurons
A type of neuron that carries nerve impulses between other neurons, often between sensory and motor neurons.
- Interphase
The longest stage in the eukaryotic cell cycle during which the cell acquires nutrients, creates and uses proteins and other molecules, and starts the process of cell division by replicating the DNA.
- Interstitial fluid
Fluid found in the spaces around cells. ... It helps bring oxygen and nutrients to cells and to remove waste products from them. As new interstitial fluid is made, it replaces older fluid, which drains towards lymph vessels. When it enters the lymph vessels, it is called lymph. Also called tissue fluid.
- Intervertebral disc
Discs which lie between adjacent vertebrae in the vertebral column. Each disc forms a fibrocartilaginous joint (a symphysis), to allow slight movement of the vertebrae, to act as a ligament to hold the vertebrae together, and to function as a shock absorber for the spine.
- Intrauterine device (IUD)
A T-shaped contraceptive structure containing copper, or a hormone that is inserted into the uterus by a physician and may be left in place for months or years.
- Intromission
The process in which a male’s penis deposits sperm in a female’s vagina.
- Involuntary
Actions which are not under one's conscious control.
- Iodine
An essential mineral commonly found in seafood. Your thyroid gland uses it to make thyroid hormones, which help control growth, repair damaged cells and support a healthy metabolism.
- Ion
An atom or molecule with a net electric charge due to the loss or gain of one or more electrons.
- Iris
A thin, annular structure in the eye, responsible for controlling the diameter and size of the pupil and thus the amount of light reaching the retina.
- Irregular bones
Bones which vary in shape and structure and therefore do not fit into any other category (flat, short, long, or sesamoid). They often have a fairly complex shape, which helps protect internal organs. For example, the vertebrae, irregular bones of the vertebral column, protect the spinal cord.
- Ischemia
An inadequate blood supply to an organ or part of the body, especially the heart muscles.
- Ischemic stroke
The most common type of stroke. It is usually caused by a blood clot that blocks or plugs a blood vessel in the brain. This keeps blood from flowing to the brain. Within minutes, brain cells begin to die. Another cause is stenosis, or narrowing of the artery.
- Isometric muscle contraction
Referring to a muscle contraction in which muscle tension increases but muscle length remains the same.
- Isotonic muscle contraction
Referring to a muscle contraction in which muscle length decreases but muscle tension remains the same.
- Isotopes
Variants of a type of atom which differ in the number of neutrons in the nucleus and therefore differ in atomic mass.
- Jejunum
One of three sections that make up the small intestine. The jejunum is located between the duodenum and the ileum.The jejunum makes up about two-fifths of the small intestine. The main function of the jejunum is absorption of important nutrients such as sugars, fatty acids, and amino acids.
- Joint
A structure where two or more bones of the skeleton come together.
- Keratin
A tough, fibrous protein in skin, hair, and nails.
- Keratinocytes
A type of epithelial cell found in the skin, hair, and nails that produces keratin.
- Kidney
One of a pair of organs of the excretory and urinary systems that filters wastes and excess water out of blood and forms urine.
- Kidney failure
The loss of the ability of nephrons in the kidney to function fully due to a progressive kidney disease such as diabetic nephropathy or polycystic kidney disease.
- Kidney stone
A solid crystal that forms in a kidney from minerals such as calcium in urine.
- Killer T Cell (cytotoxic T cell)
A T lymphocyte (a type of white blood cell), also known as a cytoxic T cell) that kills cancer cells, cells that are infected (particularly with viruses), or cells that are damaged in other ways.
- Kingdom
A major category in the classification of living things. It ranks below domain and above phylum.
- Krebs cycle (citric acid cycle)
A series of chemical reactions used by all aerobic organisms to release stored energy through the oxidation of acetyl-CoA derived from carbohydrates, fats, and proteins.
- Kyphosis
An excessive outward curvature of the spine, causing hunching of the back.
- Labia (singular: labium)
The “lips” of the vulva, consisting of folds of tissue that protect the urethral and vaginal openings.
- Labor
A general term for the process of childbirth, which includes three stages: dilation of the cervical canal, birth of the child, and delivery of the placenta (afterbirth).
- Lactation
The production of breastmilk to feed an infant.
- Lacteal
A lymphatic capillary that absorbs dietary fats in the villi of the small intestine.
- Lactic acid fermentation
A metabolic process by which glucose and other six-carbon sugars are converted into cellular energy and the metabolite lactate, which is lactic acid in solution.
- Lacunae
A small space containing an osteocyte in bone or chondrocyte in cartilage.
- Langerhans cell
A cell found in the epidermis that functions as an antigen-presenting cell which binds antigens entering through the skin.
- Large intestine
An organ of the digestive system that removes water and salts from food waste and forms solid feces for elimination.
- Larynx
An organ of the respiratory system between the pharynx and trachea, also called the voice box because it contains the vocal cords that allow the production of vocal sounds.
- Lateralization
The concentration of particular functions in one hemisphere of the cerebrum of the brain.
- Law
A statement based on repeated experimental observations that describes some aspect of the world.
- Law of conservation of mass
The law of conservation of mass states that mass can neither be created nor destroyed in a chemical reaction. Thus, the amount of matter cannot change.
- Law of independent assortment
The alleles of two (or more) different genes get sorted into gametes independently of one another.
- Law of segregation
Allele pairs separate or segregate during gamete formation and randomly unite at fertilization.
- LDL (low density lipoprotein)
The form of lipoprotein in which cholesterol is transported in the blood.
- Lens
A transparent biconvex structure in the eye that, along with the cornea, helps to refract light to be focused on the retina.
- Leukemia
A group of cancers of the blood-forming tissues in bone marrow.
- Leukocyte
a colorless cell that circulates in the blood and body fluids and is involved in counteracting foreign substances and disease; a white (blood) cell. There are several types, all amoeboid cells with a nucleus, including lymphocytes, granulocytes, monocytes, and macrophages.
- Leutenizing hormone
A hormone secreted by the anterior pituitary gland that stimulates ovulation in females and the synthesis of androgen in males.
- Lewy body
Abnormal aggregations of protein that develop inside nerve cells, contributing to Parkinson's disease (PD), the Lewy body dementias (Parkinson's disease dementia and dementia with Lewy bodies), and some other disorders.
- Leydig cell
A type of cell found between seminiferous tubules in the testes that produces and secretes testosterone.
- Ligament
A band of dense fibrous connective tissue that holds bones together.
- Limbic system
A set of brain structures located on both sides of the thalamus, immediately beneath the medial temporal lobe of the cerebrum primarily in the forebrain. It supports a variety of functions including emotion, behavior, motivation, long-term memory, and olfaction. Emotional life is largely housed in the limbic system, and it critically aids the formation of memories.
- Linked genes
Genes that are likely to be inherited together because they are physically close to one another on the same chromosome.
- Lipase
A pancreatic enzyme that catalyzes the breakdown of fats to fatty acids and glycerol.
- Lipid
A substance that is insoluble in water. Examples include fats, oils and cholesterol. Lipids are made from monomers such as glycerol and fatty acids.
- Liver
An organ of digestion and excretion that secretes bile for lipid digestion and breaks down excess amino acids and toxins in the blood.
- Living donor
A person who donates one kidney or a portion of their liver or a part of their lung to someone who needs those organs to survive. Transplants from living donors have been extremely successful and most donors recover with very few complications.
- Locus (plural: loci)
A specific, fixed position on a chromosome where a particular gene or genetic marker is located.
- Long bone
A hard, dense bone that provide strength, structure, and mobility. The thigh bone (femur) is an example of a long bone. A long bone has a shaft and two ends.
- Loop of Henle (also called loop of the nephron)
The part of a kidney tubule which forms a long loop in the medulla of the kidney, from which water and salts are resorbed into the blood.
- Lower GI tract
The part of the GI tract that includes the small and large intestines.
- Lower respiratory tract
Refers to following airway structures: trachea (windpipe) and lungs with its substructures bronchi, bronchioles, and alveoli.
- Lumbar vertebrae
Any of the five vertebrae situated between the thoracic vertebrae above and the sacrum below.
- Lung cancer
A malignant tumor characterized by uncontrolled cell growth in tissues of the lung.
- Lungs
Two paired organs of the respiratory system in which gas exchange takes place between the blood and the atmosphere.
- Lunula
The white area at the base of a fingernail.
- Luteal phase
The later phase of the ovarian cycle. It begins with the formation of the corpus luteum and ends in either pregnancy or degeneration of the corpus luteum.
- Luteinizing hormone
A hormone secreted by the anterior pituitary gland that stimulates ovulation in females and the synthesis of androgen in males.
- Lymph
A fluid that leaks out of capillaries into spaces between cells and circulates in the vessels of the lymphatic system.
- Lymph node
One of many small structures located along lymphatic vessels where pathogens are filtered from lymph and destroyed by lymphocytes.
- Lymphatic system
A body system consisting of a network of tissues and organs that help rid the body of toxins, waste and other unwanted materials. The primary function of the lymphatic system is to transport lymph, a fluid containing infection-fighting white blood cells, throughout the body.
- Lymphocyte
A type of leukocyte produced by the lymphatic system that is a key cell in the adaptive immune response to a specific pathogen or tumor cell.
- Lymphoma
A cancer that begins in infection-fighting cells of the immune system, called lymphocytes.
- Lysosome
An organelle in the cytoplasm of eukaryotic cells containing digestive enzymes enclosed in a membrane.
- Macromolecule
A very large molecule, such as protein, commonly created by the polymerization of smaller subunits (monomers).
- Macrophage
A large phagocytic cell found in stationary form in the tissues or as a mobile white blood cell, especially at sites of infection.
- Major histocompatibility complex (MHC)
A set of molecules normally found on most human cells that provide a way for the immune system to recognize body cells as self.
- Malabsorption
The imperfect absorption of food material by the small intestine.
- Mammary gland
An exocrine gland in humans and other mammals that produces milk to feed young offspring. Mammals get their name from the Latin word mamma, "breast".
- Mast cell
A type of white blood cell found in connective tissues all through the body, especially under the skin, near blood vessels and lymph vessels, in nerves, and in the lungs and intestines. Mast cells play an important role in how the immune system responds to certain pathogens by releasing chemicals such as histamines and cytokines during allergic reactions and certain immune responses.
- Matrix (of the mitochondria)
In the mitochondrion, the matrix is the space within the inner membrane. The word "matrix" stems from the fact that this space is viscous, compared to the relatively aqueous cytoplasm.
- Matter
Anything that takes up space and has mass.
- Mechanical barriers of the immune system
A physical barrier which pathogens cannot cross, protecting the body. These barriers include: The outer layer of the skin and mucous membranes.
- Mechanical digestion
The physical breakdown of chunks of food into smaller pieces by organs of the digestive system, for example chewing food.
- Mechanoreceptor
A type of sensory receptor that responds to mechanical forces.
- Medulla oblongata
A long stem-like structure which makes up part of the brainstem. It is anterior and partially inferior to the cerebellum. It is responsible for autonomic (involuntary) functions ranging from vomiting to sneezing.
- Meiosis
A special type of cell division in sexually-reproducing organisms used to produce the gametes, such as sperm or egg cells. It involves two rounds of division that ultimately result in four cells with only one copy of each chromosome.
- Melanin
A brown pigment produced by melanocytes in the skin that gives skin most of its color and prevents UV light from penetrating the skin.
- Melanocyte
A special skin cell that is responsible for producing melanin.
- Melanoma
A rare but most serious type of skin cancer that affects melanocytes and usually metastasizes if not treated.
- Melanosome
A small organelle in a melanocyte that synthesizes, stores, and transports melanin.
- Melatonin
A hormone that regulates the sleep–wake cycle, primarily released by the pineal gland.
- Membrane potential
The difference in electric potential between the interior and the exterior of a cell due to differences in the concentrations of ions on opposite sides of a cellular membrane.
- Memory cell
A lymphocyte (B or T cell) that retains a “memory” of a specific pathogen after an infection is over and thus provides immunity to the pathogen.
- Menarche
The beginning of menstruation; first monthly period in a female.
- Mendel's laws of inheritance
Consists of two laws: Mendel's Law of Segregation states individuals possess two alleles and a parent passes only one allele to his/her offspring. Mendel's Law of Independent Assortment states the inheritance of one pair of factors ( genes ) is independent of the inheritance of the other pair.
- Mendelian inheritance
A type of biological inheritance that follows the principles originally proposed by Gregor Mendel in 1865 and 1866, re-discovered in 1900 and popularised by William Bateson.
- Meninges
A three-layered membrane that encloses and protects the brain and spinal cord and contains cerebrospinal fluid.
- Menopause
The cessation of a woman’s menstrual cycles, usually by age 52.
- Menstrual cycle
The monthly cycle of processes and events in the ovaries and uterus of a sexually mature human female until menopause.
- Menstruation
The process in which the endometrium of the uterus is shed from the body during the first several days of the menstrual cycle; also called monthly period or menses.
- Merkel cell
Oval-shaped mechanoreceptors essential for light touch sensation and found in the skin.
- Messenger RNA
A large family of RNA molecules that convey genetic information from DNA to the ribosome, where they specify the amino acid sequence of the protein products of gene expression.
- Metabolism
The chemical processes that occur in a living organism to sustain life.
- Metaphase
A stage of mitosis in the eukaryotic cell cycle in which condensed chromosomes align in the equator of the cell before being separated into each of the two daughter cells.
- Metastasis
A new cancer that forms at a distant site when cancer cells from a primary tumor travel through the bloodstream.
- Microglial cells
A specialized population of macrophages found in the central nervous system (CNS). They remove damaged neurons and infections and are important for maintaining the health of the CNS.
- Microorganism
An organisms that is so small it is invisible to the human eye.
- Microvilli (singular, microvillus)
One of many tiny projections covering each villus in the mucosal lining the small intestine that increases its absorptive surface.
- Midpiece of sperm
The central part of the sperm cell between the head and the tail.
- Mitochondria (singular: mitochondrion)
A double-membrane-bound organelle found in most eukaryotic organisms. Mitochondria convert oxygen and nutrients into adenosine triphosphate (ATP). ATP is the chemical energy "currency" of the cell that powers the cell's metabolic activities.
- Mitosis
A part of the cell cycle when replicated chromosomes are separated into two new nuclei and then subsequent cell division gives rise to genetically identical cells in which the number of chromosomes is maintained.
- Mixed nerve
Nerve of the peripheral nervous system that contains both sensory and motor neurons so it can transmit signals to and from the central nervous system.
- Molar
One of twelve teeth with cusps in the back of the mouth behind the premolars used for crushing and grinding food.
- Molecule
A molecule is an electrically neutral group of two or more atoms held together by chemical bonds.
- Monomer
A molecule that can undergo polymerization, creating macromolecules. Large numbers of monomers combine to form polymers in a process called polymerization.
- Monosaccharide
The simplest form of sugar and the most basic units of carbohydrates, also called simple sugars.
- Monozygotic
Relating to twins, derived from a single ovum, and so identical.
- Motor nerve
Nerve of the peripheral nervous system that transmits information from the central nervous system to muscles, organs, and glands.
- Motor neuron
A type of neuron that carries nerve impulses from the central nervous system to muscles and glands; also called efferent neuron.
- Mouth
The opening in the lower part of the human face, surrounded by the lips, through which food is taken in and from which speech and other sounds are emitted.
- Movable joint
A joint in which the opposing bony surfaces are covered with a layer of hyaline cartilage or fibrocartilage and in which some degree of free movement is possible.
- mRNA
A large family of RNA molecules that convey genetic information from DNA to the ribosome, where they specify the amino acid sequence of the protein products of gene expression.
- Mucociliary escalator
Term for the apparatus of mucus and cilia responsible for movement of mucus up and out of the respiratory tract. Mucus traps particles and cilia propel mucus up and out of the lungs where the fluid and mucus is then swallowed and the debris eliminated by the digestive system.
- Mucosa (digestive tract)
The innermost tunic of the wall. It lines the lumen of the digestive tract. The mucosa consists of epithelium, an underlying loose connective tissue layer called lamina propria, and a thin layer of smooth muscle called the muscularis mucosa.
- Mucous membrane
Epithelial tissue that lines inner body surfaces and body openings and produces mucus.
- Mucus
A slimy substance produced by mucous membranes that traps pathogens, particles, and debris.
- Multiple allele traits
Traits controlled by a single gene with more than two alleles.
- Multiple sclerosis
A chronic, typically progressive autoimmune disease involving damage to the sheaths of nerve cells in the brain and spinal cord, whose symptoms may include numbness, impairment of speech and of muscular coordination, blurred vision, and severe fatigue.
- Muscle contraction
Increase in the tension or decrease in the length of a muscle that occurs when muscle fibers receive stimulation from the nervous system.
- Muscle fascicles
A bundle of skeletal muscle fibers surrounded by perimysium, a type of connective tissue.
- Muscle fiber
A long, thin muscle cell that has the ability to contract.
- Muscle strain
An injury in which muscle fibers tear due to overstretching of a muscle.
- Muscular dystrophy
A genetic neuromuscular disorder caused by defective proteins in muscle cells and characterized by death of skeletal muscles and progressive weakness.
- Muscular system
The body system responsible for the movement of the human body. Attached to the bones of the skeletal system are about 700 named muscles that make up roughly half of a person's body weight. Each of these muscles is a discrete organ constructed of skeletal muscle tissue, blood vessels, tendons, and nerves.
- Muscular tissue
A soft tissue that composes muscles in animal bodies, and gives rise to muscles' ability to contract. This is opposed to other components or tissues in muscle such as tendons or perimysium.
- Muscularis externa (digestive tract)
Consists of smooth muscle in most of the digestive tract, but is striated muscle in the upper part of the esophagus. Along most of the tract the externa consists of an inner circular and outer longitudinal layer.
- Musculoskeletal disorder
An injury to muscles or tendons caused by biomechanical stresses.
- Musculoskeletal system
A body system which provides form, support, stability, and movement to the body. It is made up of the bones of the skeleton, muscles, cartilage, tendons, ligaments, joints, and other connective tissue that supports and binds tissues and organs together.
- Mutagen
A physical or chemical agent that changes the genetic material, usually DNA, of an organism.
- Mutation
An alteration in the nucleotide sequence of the genome of an organism.
- Myasthenia gravis
A genetic neuromuscular disorder caused by the immune system blocking or destroying acetylcholine receptors on muscle cells and characterized by progressive muscle weakness and fatigue.
- Myelin sheath
The lipid layer around the axon of a neuron that allows nerve impulses to travel more rapidly down the axon.
- Myocardial infarction (MI)
Damage to heart muscle from death of myocardial cells that occurs when blood flow is blocked to part of the heart; also called heart attack.
- Myocardium
The muscular tissue of the heart.
- Myocyte
A type of muscle cell that makes up smooth muscle tissue.
- Myofibril
Long filaments that run parallel to each other to form muscle (myo) fibers. The muscle fibers are single multinucleated cells that combine to form the muscle. Myofibrils are made up of repeating subunits called sarcomeres.
- Myoglobin
A red protein containing heme, which carries and stores oxygen in muscle cells. It is structurally similar to a subunit of hemoglobin.
- Myokine
One of several hundred cytokines or other small proteins produced and released by muscle cells (myocytes) in response to muscular contractions with an endocrine function.
- Myometrium
The middle layer of the uterine wall, consisting mainly of uterine smooth muscle cells (also called uterine myocytes) but also of supporting stromal and vascular tissue. Its main function is to induce uterine contractions.
- Myopia
A vision problem in which distant objects are out of focus but close vision is unaffected; also called nearsightedness.
- Myosin
A fibrous protein that forms (together with actin) the contractile filaments of muscle cells and is also involved in motion in other types of cells.
- Nail
accessory organ of the skin made of sheets of dead keratinocytes at the distal ends of the fingers and toes
- Nail bed
The pink skin under the nail plate visible through the nail.
- Nail fold
The tissue that encloses the nail matrix at the root of the nail.
- Nail matrix
A deep layer of epidermal tissue at the proximal end of a nail where nail growth occurs.
- Nail plate
visible part of a nail that is external to the skin
- Nail root
A portion of a nail that is under the surface of the skin at the proximal end of the nail.
- Nasal cavity
A large, air-filled space in the skull above and behind the nose that helps conduct air in and out of the body as part of the upper respiratory tract.
- Natural killer cell
A type of immune cell that has granules (small particles) with enzymes that can kill tumor cells or cells infected with a virus. A natural killer cell is a type of white blood cell.
- Natural selection
The differential survival and reproduction of individuals due to differences in phenotype. It is a key mechanism of evolution, the change in the heritable traits characteristic of a population over generations.
- Neanderthal
An extinct species or subspecies of archaic humans who lived in Eurasia until about 40,000 years ago. They probably went extinct due to competition with or extermination by immigrating modern humans or due to great climatic change, disease, or a combination of these factors.
- Negative feedback
A control mechanism that serves to reduce an excessive response and keep a variable within its normal range.
- Negative feedback loop
A control mechanism that serves to reduce an excessive response and keep a variable within its normal range.
- Nephron
One of the million tiny structural and functional units of the kidney that filters blood and forms urine.
- Nerve
A structure in the nervous system that consists of cable-like bundles of axons and makes up the majority of the peripheral nervous system.
- Nerve impulse
A signal transmitted along a nerve fiber.
- Nervous system
The highly complex body system of an animal that coordinates its actions and sensory information by transmitting signals to and from different parts of its body. The nervous system detects environmental changes that impact the body, then works in tandem with the endocrine system to respond to such events.
- Nervous tissue
A specialized tissue found in the central nervous system and the peripheral nervous system. It consists of neurons and supporting cells called neuroglia. The nervous system is responsible for the control of the body and the communication among its parts.
- Neurochemical
A neurotransmitter or other chemical substance that affects the nervous system.
- Neurodegenerative disorder
A type of disease in which cells of the central nervous system stop working or die. Neurodegenerative disorders usually get worse over time and have no cure. They may be genetic or be caused by a tumor or stroke.
- Neurogenesis
The formation of new neurons by cell division.
- Neuroglia
A class of nervous system cell that provides support for neurons and helps them transmit nerve impulses.
- Neuroimmune system
A part of the immune system that protects the central nervous system.
- Neuromuscular disorder
A disorder that occurs due to problems with the nervous control of muscle contractions or with muscle cells themselves.
- Neuromuscular junction
A chemical synapse where a motor neuron transmits a signal to a muscle fiber to initiate a muscle contraction.
- Neuron
A functional unit of the nervous system that transmits nerve impulses; also called a nerve cell.
- Neurotransmitter
A type of chemical that transmits signals from the axon of a neuron to another cell across a synapse.
- Neutrons
A sub-atomic particle with a charge of 0.
- Neutrophil
A type of immune cell that is one of the first cell types to travel to the site of an infection. Neutrophils help fight infection by ingesting microorganisms and releasing enzymes that kill the microorganisms. A neutrophil is a type of white blood cell, a type of granulocyte, and a type of phagocyte.
- Nicotine
A highly addictive psychoactive stimulant drug that is found in tobacco and tobacco smoke.
- Nipple
A raised, coloured region of tissue on the surface of the breast from which, in females, milk leaves the breast through the lactiferous ducts to feed an infant.
- Nociceptor
A type of sensory receptor that responds to pain.
- Nodes of Ranvier
One of the regularly spaced gaps in the myelin sheath along an axon that allows the action potential (electrical signal) to travel very rapidly.
- Non-Mendelian inheritance
Any pattern of inheritance in which traits do not segregate in accordance with Mendel's laws. This includes inheritance of multiple allele traits, codominance, incomplete dominance and polygenic traits.
- Non-self proteins
Foreign proteins on the surface of a cell that triggers an immune response.
- Non-steroid hormone
Any type of endocrine hormone that is made of amino acids and binds with a receptor on the plasma membrane of a target cell.
- Nondisjunction
The failure of homologous chromosomes or sister chromatids to separate properly during cell division.
- Noonan Syndrome with Multiple Lentigines
A rare genetic disorder characterized by abnormalities of the skin, the structure and function of the heart, the inner ear, the head and facial (craniofacial) area, and/or the genitals.
- Noradrenaline
A substance that is released predominantly from the ends of sympathetic nerve fibres and that acts to increase the force of skeletal muscle contraction and the rate and force of contraction of the heart.
- Normal range
The spread of values around the set point of a biological variable such as body temperature that is considered normal, with no negative effects on health.
- Norovirus
Any of various single-stranded RNA viruses including the Norwalk virus and closely related viruses which cause gastroenteritis.
- NSAIDs
Non-steroidal anti-inflammatory drugs; ex. ibuprofen.
- Nuclear envelope
A structure made up of two lipid bilayer membranes which in eukaryotic cells surrounds the nucleus, which encases the genetic material. Also know as the nuclear membrane.
- Nuclear force
A force that acts between the protons and neutrons of atoms.
- Nuclear pore
A protein-lined channel in the nuclear envelope that regulates the transportation of molecules between the nucleus and the cytoplasm.
- Nucleic acids
Large biomolecules, essential to all known forms of life. The term nucleic acid is the overall name for DNA and RNA. They are composed of nucleotides, which are the monomers made of three components: a 5-carbon sugar, a phosphate group and a nitrogenous base.
- Nucleolus
A structure in the nucleus of eukaryotic cells which is the site of ribosome synthesis/production.
- Nucleoplasm
A solution, similar to the cytoplasm of a cell, enveloped by the nuclear envelope and surrounding the chromosomes and nucleolus.
- Nucleotide
One of the structural components, or building blocks, of DNA and RNA. A nucleotide consists of a base (one of four chemicals: adenine, thymine, guanine, and cytosine) plus a molecule of sugar and one of phosphoric acid.
- Nucleus
A central organelle containing hereditary material.
- Obesity
Abnormal or excessive fat accumulation that presents a risk to health. Obesity has been more precisely defined by the National Institutes of Health (the NIH) as a BMI (Body Mass Index) of 30 and above.
- Observation
Receiving knowledge of the outside world through our senses, or recording information using scientific tools and instruments.
- Occipital lobe
A part of each hemisphere of the cerebrum that is dedicated almost solely to vision.
- Oligodendrocyte
A type of neuroglia whose main functions are to provide support and insulation to axons in the central nervous system of some vertebrates, equivalent to the function performed by Schwann cells in the peripheral nervous system.
- Oocyte
A cell in an ovary which may undergo meiotic division to form an ovum.
- Oogenesis
The production or development of an ovum.
- Oogonium (plural: oogonia)
A diploid stem cell in an ovary that undergoes mitosis to begin the process of oogenesis.
- Opioids
A class of drug derived from the opium poppy or a synthetic version of such a drug, including heroin and painkillers such as codeine, morphine, or OxyContin.
- Optic disc
The point of exit for ganglion cell axons leaving the eye. Because there are no rods or cones overlying the optic disc, it corresponds to a small blind spot in each eye. The ganglion cell axons form the optic nerve after they leave the eye.
- Organ
A group of tissues in a living organism that have been adapted to perform a specific function. In higher animals, organs are grouped into organ systems; e.g., the esophagus, stomach, and liver are organs of the digestive system.
- Organ system
A group of organs that work together to perform one or more functions. Each does a particular job in the body, and is made up of certain tissues.
- Organelle
A tiny cellular structure that performs specific functions within a cell.
- Organism
An individual living thing.
- Osmosis
The movement of water or other solvent through a plasma membrane from a region of low solute concentration to a region of high solute concentration.
- Ossification
The process in which cartilage is changed into bone.
- Osteoarthritis
The degeneration of joint cartilage and the underlying bone, most common from middle age onward. It causes pain and stiffness, especially in the hip, knee, and thumb joints.
- Osteoblast
A type of bone cell that makes and mineralizes bone matrix.
- Osteocalcin
An endocrine hormone secreted by bone cells that helps to regulate blood glucose and fat deposition.
- Osteoclast
A type of bone cell that breaks down bone, dissolves its minerals, and releases them into the blood.
- Osteocyte
A type of bone cell that helps regulate the formation and breakdown of bone tissue.
- Osteogenic cell
A type of stem cell that can divide and differentiate to form new bone cells.
- Osteoporosis
A medical condition in which the bones become brittle and fragile from loss of tissue, typically as a result of hormonal changes, or deficiency of calcium or vitamin D.
- Ovarian cycle
The series of events of the menstrual cycle that occur in the ovaries, including maturation of a follicle, ovulation, and development of the corpus luteum.
- Ovarian follicle
The functional unit of an ovary that consists of a nest of epithelial cells surrounding an egg.
- Ovaries
A pair of female reproductive organs that produces eggs and secretes estrogen.
- Oviduct (also known as Fallopian tubes)
One of two female reproductive organs that carry eggs from an ovary to the uterus and are the site where fertilization usually takes place.
- Ovulation
The release of a secondary oocyte from an ovary about half way through the menstrual cycle.
- Ovum (plural: ova)
The gamete produced by a female.
- Oxytocin
An endocrine hormone secreted by the pituitary gland that controls a variety of functions, including during childbirth to stimulate uterine contractions and during lactation to trigger milk letdown.
- Pacemaker cells
A type of cells located in the heart that create electrical signals to stimulate heart muscles to contract.
- Pancreas
A long, flat gland that sits tucked behind the stomach in the upper abdomen. The pancreas produces enzymes that help digestion and hormones that help regulate the way your body processes sugar (glucose).
- Pancreatic duct
The excretory duct of the pancreas, extending through the gland from tail to head, where it empties into the duodenum.
- Pancreatic islets
One of millions of clusters of cells in the pancreas that secrete endocrine hormones such as insulin and glucagon; also called islet of Langerhans.
- Pancreatic polypeptide
A non-steroid hormone which helps the pancreas self-regulate secretion.
- Pancreatitis
A painful inflammation of the pancreas due to gallstones, chronic alcohol use, or other cause.
- Pap smear
A medical test in which cells are scraped from the cervix and examined under a microscope in order to detect cancer cells, or precancerous cells, if they are present.
- Papillae
Nodules on the surface of the tongue that increase the surface area for the taste buds. Not all papillae, however, contain taste buds. The papillae also appear to aid in the mechanical handling of food, providing a rough surface.
- Papillary layer of the dermis
The upper layer of the dermis with papillae extending upward into the epidermis.
- Papillary muscle
One of the small bundles of muscles attached to the ventricle walls and to the chordae tendineae that tighten these tendons during ventricular contraction.
- Parafollicular cells
Neuroendocrine cells in the thyroid. The primary function of these cells is to secrete calcitonin. They are located adjacent to the thyroid follicles and reside in the connective tissue.
- Paralysis
The loss of sensation and movement in part of the body, such as may occur with a stroke or spinal cord injury.
- Parasite
A species that lives in or on another species, called the host, and causes harm to the host while benefitting from the relationship.
- parasites
- Parasympathetic division
The division of the autonomic nervous system that returns the body to normal after the fight-or-flight response and maintains homeostasis at other times.
- Parathyroid gland
One of a pair of small endocrine glands in the neck that secretes hormones that regulate blood calcium.
- Parathyroid hormone
A hormone secreted by the parathyroid gland which helps regulate blood calcium.
- Parietal lobe
The part of each hemisphere of the cerebrum that is involved in functions such as touch, reading, and arithmetic.
- Parkinson’s disease
A degenerative brain disorder caused by progressive death of neurons in the midbrain, resulting in muscular symptoms of tremor, rigidity, slowness of movement, and postural instability.
- Parotid gland
Either of a pair of large salivary glands situated just in front of each ear.
- Partly movable joint
An articulation between bones (also called an amphiarthrotic joint) in which the motion is limited due to either fibrous tissue or cartilage.
- Passive immunity
Short-term immunity to a particular pathogen that results when antibodies or activated T cells are transferred to a person who has never been exposed to the pathogen.
- Passive transport
a type of movement of substances across the cell membrane which does not require energy because the substances are moving with the concentration gradient (from high to low concentration).
- Pathogen
A microorganism which causes disease.
- Pectoral girdle (also known as the shoulder girdle)
Paired clavicles (collar bones) and scapulas (shoulder blades) that together form the shoulders and attach the arms to the trunk; also called shoulder girdle.
- Pedigree
A diagram that shows the occurrence and appearance of phenotypes of a particular gene or organism and its ancestors from one generation to the next, most commonly humans, show dogs, and race horses.
- Pelvic cavity
A body cavity that is bounded by the bones of the pelvis. Its oblique roof is the pelvic inlet (the superior opening of the pelvis). Its lower boundary is the pelvic floor. The pelvic cavity primarily contains reproductive organs, the urinary bladder, the pelvic colon, and the rectum.
- Pelvic girdle
Paired, fused bones (ilium, pubis, and ischium) that form the hips and attach the legs to the trunk.
- Penis
The male reproductive organ containing the urethra, through which semen and urine pass out of the body.
- Pepsin
The chief digestive enzyme in the stomach, which breaks down proteins into polypeptides.
- Peptic ulcer
A sore that develops in the lining of the stomach or duodenum most often caused by infection with the bacterium Helicobacter pylori.
- Peptidase
An enzyme which breaks down peptides into amino acids.
- Pericardium
The membrane enclosing the heart, consisting of an outer fibrous layer and an inner double layer of serous membrane.
- Perimetrium
The outer serous layer of the uterus. The serous layer secretes a lubricating fluid that helps to reduce friction.
- Perimysium
The sheath of connective tissue surrounding a bundle of muscle fibers.
- Periosteum
A tough, fibrous membrane that covers the outer surface of bones.
- Peripheral artery disease (PAD)
The narrowing of peripheral arteries, usually in the legs, due to atherosclerosis and generally causing intermittent pain in the legs when walking.
- Peripheral immune system
The part of the immune system that protects all of the body except for the central nervous system (which is protected by the neuroimmune system).
- Peripheral nervous system
One of two major divisions of the nervous system that consists of all the nervous tissue that lies outside the central nervous system.
- Peristalsis
A distinctive pattern of smooth muscle contractions that propels foodstuffs distally through the esophagus and intestines.
- Peritubular capillary network
Tiny blood vessels, supplied by the efferent arteriole, that travel alongside nephrons allowing reabsorption and secretion between blood and the inner lumen of the nephron.
- pH
A measure of the acidity or basicity of aqueous or other liquid solutions. The term translates the values of the concentration of the hydrogen ion in a scale ranging from 0 and 14. In pure water, which is neutral (neither acidic nor alkaline), the concentration of the hydrogen ion corresponds to a pH of 7. A solution with a pH less than 7 is considered acidic; a solution with a pH greater than 7 is considered basic, or alkaline.
- PH scale
A scale used to specify how acidic or basic a water-based solution is. Acidic solutions have a lower pH, while basic solutions have a higher pH.
- Phagocytosis
The process by which a cell uses its plasma membrane to engulf a large particle, giving rise to an internal compartment called the phagosome.
- Pharmacogenomics
The study of how our genes affect the way we respond to drugs.
- Pharynx
Tubular organ that connects the mouth and nasal cavity with the larynx and through which air and food pass.
- Phenotype
The set of observable characteristics of an individual resulting from the interaction of its genotype with the environment.
- Pheomelanin
A lighter pigment found in red hair, and is concentrated in the redder areas of the skin such as the lips. Because people with red hair are less able to make the dark eumelanin pigment, their skin is generally quite pale and burns easily with sun exposure.
- Pheromone
A secreted or excreted chemical factor that triggers a social response in members of the same species. Pheromones are chemicals capable of acting like hormones outside the body of the secreting individual, to impact the behavior of the receiving individuals.
- Phospholipid
A type of biological molecule consisting of two hydrophobic fatty acid "tails" and a hydrophilic "head" consisting of a phosphate group. Phospholipids are a major component of all cell membranes.
- Phospholipid bilayer
A thin polar membrane made of two layers of phospholipid molecules. These membranes are flat sheets that form a continuous barrier around all cells.
- Phosphorus
The second most plentiful mineral in your body. The first is calcium. Your body needs phosphorus for many functions, such as filtering waste and repairing tissue and cells.
- Photoreceptor
A type of sensory receptor that responds to light.
- Photosynthesis
Photosynthesis is a process used by plants and other organisms to convert light energy into chemical energy that can later be released to fuel the organisms' activities.
- Phylogenetic tree
A tree diagram used to show the hypothesized evolutionary relationships between groups of organisms.
- Phylogeny
The evolutionary development and history of a species or trait of a species or of a higher taxonomic grouping of organisms.
- Physical exercise
Any bodily activity that enhances or maintains physical fitness and good health.
- Physiology
The study of the functioning of the human organism.
- Pineal gland
An endocrine gland that secretes the hormone melatonin, which regulates the sleep-wake cycle.
- Pinocytosis
The ingestion of liquid into a cell by the budding of small vesicles from the cell membrane.
- Pituitary gland
The master gland of the endocrine system that secretes many hormones, the majority of which regulate other endocrine glands.
- Pivot joint
The joints that allow bones to rotate. In a pivot joint, a cylinder shaped bone rotates inside another bone or ligament that forms a ring around it.
- Placenta
A temporary organ that consists of a large mass of maternal and fetal blood vessels through which the mother’s and embryo’s or fetus’s blood exchange substances.
- Plane joint
A type of structure in the body, also called gliding joint, formed between two bones in which the articular, or free, surfaces of the bones are flat or nearly flat, enabling the bones to slide over each other.
- Plasma
A straw-yellow fluid part of blood that contains many dissolved substances and blood cells.
- Plasma cell
A fully differentiated B cell that produces a single type of antibody.
- Plasma membrane
A semi-permeable lipid bilayer that separates the interior of all cells from their surroundings.
- Pleiotropy
Describes the genetic effect of a single gene on multiple phenotypic traits. The underlying mechanism is genes that code for a product that is either used by various cells or has a cascade-like signaling function that affects various targets.
- Pleura
Each of a pair of serous membranes lining the thorax and enveloping the lungs.
- Pneumonia
A disease in which the alveoli of the lungs become inflamed and filled with fluid, usually as a result of infection, causing symptoms such as shortness of breath, coughing, chest pain, and fever.
- Point mutation
A mutation that only affects a single nucleotide of nucleic acid.
- Polarity
A separation of electric charge leading to a molecule or its chemical groups having a negatively charged end and a positively charged end.
- Pollination
The act of transferring pollen grains from the male anther of a flower to the female stigma.
- Polyadenylation
The addition of a poly(A) tail to a messenger RNA. The poly(A) tail consists of multiple adenosine monophosphates.
- Polycystic kidney disease (PKD)
A genetic disorder in which multiple abnormal cysts develop and grow in the kidneys.
- Polycystic ovary syndrome (PCOS)
a hormonal disorder common among women of reproductive age. Women with PCOS may have infrequent or prolonged menstrual periods or excess male hormone (androgen) levels. The ovaries may develop numerous small collections of fluid (follicles) and fail to regularly release eggs.
- Polygenic traits
One whose phenotype is influenced by more than one gene. Traits that display a continuous distribution, such as height or skin color, are polygenic.
- Polymer
A large molecule, or macromolecule, composed of many repeated subunits.
- Polymerase chain reaction (PCR)
A method used widely in molecular biology to make millions to billions of copies of a specific DNA sample rapidly, allowing scientists to take a very small sample of DNA and amplify it to a large enough amount to study in detail.
- Polymorphism
A discontinuous genetic variation resulting in the occurrence of several different forms or types of individuals among the members of a single species.
- Polynucleotide
A polymer composed of 13 or more nucleotide monomers covalently bonded in a chain. DNA and RNA are examples of polynucleotides with distinct biological function.
- Polysaccharide
Polysaccharides are carbohydrate molecules composed of long chains of monosaccharide units bound together. They range in structure from linear to highly branched.
- Pons
Part of the central nervous system, located at the base of the brain, between the medulla oblongata and the midbrain. It is part of the brainstem. The pons serves as a message station between several areas of the brain. It helps relay messages from the cortex and the cerebellum
- Positive feedback loop
A control mechanism that serves to intensify a response until an endpoint is reached.
- Posterior pituitary gland
The back lobe of the pituitary gland that stores and secretes hypothalamic hormones.
- Postsynaptic cell
The cell that receives the nerve impulse.
- Pregnancy
The carrying of one or more offspring from fertilization until birth.
- Premolar
One of eight cusped teeth in the sides of the jaws between the canine teeth and molars that are used for crushing food.
- Presbyopia
Farsightedness caused by loss of elasticity of the lens of the eye, occurring typically in middle and old age.
- Presynaptic cell
The cell that sends the nerve impulse.
- Primary immunodeficiency
A group of more than 400 rare, chronic disorders in which part of the body’s immune system is missing or functions improperly. While not contagious, these diseases are caused by hereditary or genetic defects, and, although some disorders present at birth or in early childhood, the disorders can affect anyone, regardless of age or gender.
- Primary lymphoid organ
Any organ where lymphocytes are formed and mature. They provide an environment for stem cells to divide and mature into B- and T- cells: There are two primary lymphatic organs: the red bone marrow and the thymus gland.
- Primary ossification center
The first area of a bone to start ossifying. It usually appears during prenatal development in the central part of each developing bone. In long bones the primary centers occur in the diaphysis/shaft and in irregular bones the primary centers occur usually in the body of the bone.
- Primate
A groups of mammals characterized by flexible hands and feet, each with five digits, including humans, great apes, monkeys, and lemurs.
- Producers (also known as autotrophs)
Organisms that make their own food. They get energy from chemicals or the sun, and with the help of water, convert that energy into useable energy in the form of sugar, or food. The most common example of a producer are plants.
- Product
A substance that is formed as the result of a chemical reaction.
- Progesterone
The female sex hormone secreted mainly by the ovaries that helps maintain a successful pregnancy.
- Prokaryotic
Cells which lack membrane-bound structures, specifically a nucleus. Instead they generally have a single circular chromosome located in an area of the cell called the nucleoid.
- Prolactin
The hormone that tells the body to make breast milk when a person is pregnant or breast-feeding. Production of prolactin takes place in the pituitary gland. For those who are not pregnant or breast-feeding, there are only low levels of prolactin in the body.
- Prolapse
In medicine, prolapse is a condition in which organs fall down or slip out of place. It is used for organs protruding through the vagina, rectum, or for the misalignment of the valves of the heart.
- Proliferative phase
The second phase of the uterine cycle when estrogen causes the endometrium lining of the uterus to grow, or proliferate, during this time.
- Promoter
A sequence of DNA to which proteins bind that initiate transcription of a single mRNA from the DNA downstream of it.
- Prophase
The first stage of cell division in both mitosis and meiosis. Beginning after interphase, DNA has already been replicated when the cell enters prophase. The main occurrences in prophase are the condensation of the chromatin and the disappearance of the nucleolus.
- Prostate cancer
A tumor in the prostate gland of the male reproductive system that is the most common type of cancer in men.
- Prostate gland
A gland in the male reproductive system that secretes fluid into semen and provides nourishing substances to sperm.
- Protein
A class of biological molecule consisting of linked monomers of amino acids and which are the most versatile macromolecules in living systems and serve crucial functions in essentially all biological processes.
- Protein synthesis
The process of creating protein molecules.
- Proto-oncogenes
A normal gene which, when altered by mutation, becomes an oncogene that can contribute to cancer. Proto-oncogenes may have many different functions in the cell. Some proto-oncogenes provide signals that lead to cell division. Other proto-oncogenes regulate programmed cell death (apoptosis).
- Proton
A sub-atomic particle with a charge of +1.
- Proximal convoluted tubule
The portion of the nephron that lies between the glomerular capsule and the loop of Henle and functions especially in the resorption of sugar, sodium and chloride ions, and water from the glomerular filtrate.
- Pseudoscience
A collection of beliefs or practices mistakenly regarded as being based on scientific method.
- Psychoactive drugs
A drug that affects the central nervous system, generally by influencing neurotransmitters in the brain.
- Puberty
A period during which humans become sexually mature.
- Pulmonary
Relating to the lungs.
- Pulmonary artery
The artery carrying blood from the right ventricle of the heart to the lungs for oxygenation.
- Pulmonary circuit
The part of the cardiovascular system that carries blood between the heart and lungs.
- Pulmonary embolism
A blockage in one of the pulmonary arteries in your lungs. In most cases, pulmonary embolism is caused by blood clots that travel to the lungs from deep veins in the legs or, rarely, from veins in other parts of the body (deep vein thrombosis).
- Pulmonary semilunar valve
The semilunar valve of the heart that lies between the right ventricle and the pulmonary artery and has three cusps (sometimes referred to as the pulmonic valve).
- Pulmonary vein
A vein carrying oxygenated blood from the lungs to the left atrium of the heart.
- Punnett square
A square diagram that is used to predict the genotypes of a particular cross or breeding experiment. Named after Reginald C. Punnett, who devised the approach, this diagram is used by biologists to determine the probability of an offspring having a particular genotype.
- Pupil
A hole located in the center of the iris of the eye that allows light to strike the retina.
- Pyelonephritis
An inflammation of the substance of the kidney as a result of bacterial infection.
- Racism
The prejudice, discrimination, or antagonism directed against someone of a different race based on the belief that one's own race is superior.
- Reabsorption
The process by which the nephron removes water and solutes from the tubular fluid (pre-urine) and returns them to the circulating blood.
- Reactant
A substance that takes part in and undergoes change during a chemical reaction.
- Reading Frame
The specific location in DNA where a set of codons will code for a certain protein. The reading frame begins with the start codon (AUG).
- Receptor
A protein on a cell membrane or inside of a cell that binds with a hormone, neurotransmitter, or other chemical signal to produce a response.
- Recessive
A gene that can be masked by a dominant gene. In order to have a trait that is expressed by a recessive gene, such as blue eyes, you must get the gene for blue eyes from both of your parents.
- Rectum
A short part of the large intestine between the colon and anus where feces is stored until it is eliminated through the anus.
- Red blood cell (also known as an erythrocyte)
A type of cell in blood that contains hemoglobin and carries oxygen.
- Red bone marrow
A delicate, highly vascular fibrous tissue containing hematopoietic stem cells. These are blood-forming stem cells.
- Regulatory elements
Regions of non-coding DNA which regulate the transcription of neighboring genes.
- Regulatory protein
Any protein that influences the regions of a DNA molecule that are transcribed by RNA polymerase during the process of transcription.
- Renal capsule
A thin membranous sheath that covers the outer surface of each kidney. The capsule is composed of tough fibres, chiefly collagen and elastin (fibrous proteins), that help to support the kidney mass and protect the vital tissue from injury.
- Renal column
An extension of the renal cortex in between the renal pyramids. It allows the cortex to be better anchored. Each column consists of lines of blood vessels and urinary tubes and a fibrous material.
- Renal cortex
The outer portion of the kidney between the renal capsule and the renal medulla.
- Renal medulla
The innermost part of the kidney. The renal medulla is split up into a number of sections, known as the renal pyramids.
- Renal pelvis
The funnel-like end of a ureter where it enters the kidney and where urine collects before it is transported through the ureter.
- Renal pyramid
Any of the triangular sections of tissue that constitute the medulla, or inner substance, of the kidney. The pyramids consist mainly of tubules that transport urine from the cortical, or outer, part of the kidney, where urine is produced, to the calyces, or cup-shaped cavities in which urine collects before it passes through the ureter to the bladder.
- Renal tubule
A tubular structure of a nephron in a kidney through which filtered substances pass and where some filtered substances are reabsorbed by the blood and additional substances are secreted from the blood.
- Renin
An enzyme secreted by and stored in the kidneys which promotes the production of the protein angiotensin.
- Repressors
Regulatory proteins that prevent transcription by impeding the progress of RNA polymerase along the DNA strand, so the DNA cannot be transcribed to mRNA.
- Reproduction
The production of offspring by sexual or asexual process.
- Reproductive system
The body system by which humans reproduce and bear live offspring.
- Respiration
The exchange of gases between the body and the outside air.
- Respiratory center
One of several areas in the medulla oblongata and pons of the brain stem that help control unconscious breathing.
- Respiratory conduction
The movement of air into and out of the body.
- Respiratory system
The body system responsible for taking in oxygen and expelling carbon dioxide. The primary organs of the respiratory system are the lungs, which carry out this exchange of gases as we breathe.
- Respiratory tract
The continuous system of passages through which air flows into and out of the body.
- Resting potential
The difference in electrical charge across the plasma membrane of a neuron that is not actively transmitting a nerve impulse.
- Reticular activating system
A diffuse network of nerve pathways in the brainstem connecting the spinal cord, cerebrum, and cerebellum, and mediating the overall level of consciousness.
- Reticular layer of the dermis
The lower layer of the dermis that gives the dermis strength and elasticity and contains many dermal structures such as glands and hair follicles.
- Retina
A layer at the back of the eyeball containing cells that are sensitive to light and that trigger nerve impulses that pass via the optic nerve to the brain, where a visual image is formed.
- Rheumatoid arthritis
A chronic progressive disease causing inflammation in the joints and resulting in painful deformity and immobility, especially in the fingers, wrists, feet, and ankles.
- Rib cage
A bony “cage” enclosing the thoracic cavity and consisting of the ribs, thoracic vertebrae, and sternum.
- Ribcage
bony “cage” enclosing the thoracic cavity and consisting of the ribs, thoracic vertebrae, and sternum
- Ribose
A simple sugar and carbohydrate with molecular formula C5H10O5.
- Ribosomal RNA (also known as rRNA)
A type of RNA that acts as the primary building block for ribosomes and the assembly line on which protein synthesis occurs in those ribosomes.
- Ribosome
A large complex of RNA and protein which acts as the site of RNA translation, building proteins from amino acids using messenger RNA as a template.
- RNA (ribonucleic acid)
A nucleic acid of which many different kinds are now known, including messenger RNA, transfer RNA and ribosomal RNA.
- Rod cells
Photoreceptor cells in the retina of the eye that can function in lower light than the other type of visual photoreceptor, cone cells. Rods are usually found concentrated at the outer edges of the retina and are used in peripheral vision.
- Rotavirus
Any of a group of RNA viruses, some of which cause acute enteritis (inflammation of the lower digestive tract and possibly diarrhea) in humans.
- Rough endoplasmic reticulum
An organelle found in eukaryotic cells. Its main function is to produce proteins. It is a portion of the endoplasmic reticulum which is studded with attached ribosomes.
- rRNA (also known as ribosomal RNA)
A type of RNA that acts as the primary building block for ribosomes and the assembly line on which protein synthesis occurs in those ribosomes.
- Sacral vertebrae
A large, triangular bone at the base of the spine that forms by the fusing of sacral vertebrae S1–S5 between 18 and 30 years of age.
- Saddle joint
A type of synovial joint that allow articulation by reciprocal reception. Both bones have concave-convex articular surfaces which interlock like two saddles opposed to one another (example, the base of the thumb).
- Saliva
A fluid secreted by salivary glands that keeps the mouth moist and contains the digestive enzymes amylase and lipase.
- Salivary gland
One of many exocrine glands in the mouth that secrete saliva into the mouth through ducts.
- Sarcomere
The basic functional unit of skeletal and cardiac muscles, containing actin and myosin protein filaments that slide over one another to produce a shortening of the sarcomere resulting in a muscle contraction.
- Sarcopenia
A gradual decrease in the ability to maintain skeletal muscle mass that occurs in later adulthood.
- Saturated fatty acid
A type of fat in which the fatty acid chains have all or predominantly single bonds.
- Schistosomiasis
A disease caused by parasitic flatworms called schistosomes, also known as snail fever and bilharzia. The urinary tract or the intestines may be infected. Symptoms include abdominal pain, diarrhea, bloody stool, or blood in the urine.
- Schwann cell
A variety of neuroglia that keep peripheral nerve fibres (both myelinated and unmyelinated) alive. In myelinated axons, Schwann cells form the myelin sheath.
- Science
A large body of knowledge and the process by which this knowledge is obtained.
- Scientific investigation
The way in which scientists and researchers use a systematic approach to answer questions about the world around us.
- Scientific law
A scientific law is a statement based on repeated experimental observations that describes some aspect of the world.
- Scientific method
Principles and procedures for the systematic pursuit of knowledge involving the recognition and formulation of a problem, the collection of data through observation and experiment, and the formulation and testing of hypotheses.
- Scientific racism
A falsely held, pseudoscientific belief, sometimes termed biological racism, that empirical evidence exists to support or justify racism (racial discrimination).
- Scrotum
A pouch-like external structure of the male reproductive system, located behind the penis, that contains the testes, epididymes, and part of the vas deferens.
- Sebaceous gland
A gland in the dermis of the skin that produces sebum, an oily substance that waterproofs the skin and hair.
- Sebum
An oily secretion of the sebaceous glands.
- Secondary immunodeficiency
Occurs when the immune system is compromised due to an environmental factor. Examples of these outside forces include HIV, chemotherapy, severe burns or malnutrition.
- Secondary lymphoid organs
A set of organs which includes lymph nodes and the spleen) maintain mature naive lymphocytes and initiate an adaptive immune response.
- Secondary ossification center
The area of ossification that appears after the primary ossification center has already appeared – most of which appear during the postnatal and adolescent years. Most bones have more than one secondary ossification center. In long bones, the secondary centers appear in the epiphyses.
- Secondary sex characteristic
A trait that is different in males and females but is not directly involved in reproduction, such as male facial hair and female breasts.
- Secondhand smoke
Smoke that enters the air from burning cigarettes or from the lungs of smokers.
- Secretion
Transport which occurs in the proximal tubule section of the nephron, and is responsible for the movement of certain molecules out of the blood and into the urine.
- Secretory phase
The stage of the menstrual cycle immediately following ovulation, during which the womb lining is at full thickness and its mucus glands are actively secreting.
- Selective breeding
As artificial selection, is a process used by humans to develop new organisms with desirable characteristics. Breeders select two parents that have beneficial phenotypic traits to reproduce, yielding offspring with those desired traits.
- Selectively permeable
A membrane which allows the passage of some molecules or ions and inhibits the passage of others. The capacity to filter molecular transport in this manner is called selective permeability.
- Self molecules
Components of an organism’s body that can be distinguished from foreign substances by the immune system.
- Self-pollination
The pollination of a flower by pollen from the same flower or from another flower on the same plant.
- Semen
Fluid containing sperm and glandular secretions, which nourishes sperm and carries them through the urethra and out of the body.
- Semicircular canals
Three tiny, fluid-filled tubes in your inner ear that help you keep your balance. When your head moves around, the liquid inside the semicircular canals sloshes around and moves the tiny hairs that line each canal.
- Seminal vesicle
One of a pair of glands of the male reproductive system that secretes fluid into semen.
- Seminiferous tubules
One of the many tiny tubes contained within the testes where sperm are produced.
- Sensor
Component of a homeostatic mechanism that senses the value of a variable and sends data on it to the control center.
- Sensory nerve
Nerve of the peripheral nervous system that transmits information from sensory receptors in the body to the central nervous system.
- Sensory neuron
Type of neuron that carries nerve impulses from sensory receptors in tissues and organs to the central nervous system; also called afferent neuron.
- Sensory receptor
Specialized nerve cell that responds to a particular type of stimulus such as light or chemicals by generating a nerve impulse.
- Serosa (digestive tract)
A smooth membrane that consists of a thin connective tissue layer and a thin layer of cells that secrete serous fluid.
- Serotonin
A neurotransmitter. It has a popular image as a contributor to feelings of well-being and happiness, though its actual biological function is complex and multifaceted, modulating cognition, reward, learning, memory, and numerous physiological processes such as vomiting and vasoconstriction.
- Sertoli cell
A type of cell that lines the seminiferous tubules in the testes and plays several roles in sperm production.
- Sesamoid bones
A small independent bone or bony nodule developed in a tendon where it passes over an angular structure, typically in the hands and feet. The kneecap is a particularly large sesamoid bone.
- Set point
A physiologically optimum value for a given biological variable such as body temperature.
- Sex chromosomes
A pair of chromosomes that determines biological sex.
- Sex hormone
An endocrine hormone secreted mainly by gonads that controls sexual development and reproduction.
- Sex-linked genes
Traits in which a gene is located on a sex chromosome. In humans, the term generally refers to traits that are influenced by genes on the X chromosome.
- Sex-linked traits
A trait in which a gene is located on a sex chromosome. This is because the X chromosome is large and contains many more genes than the smaller Y chromosome. In a sex-linked disease, it is usually males who are affected because they have a single copy of X chromosome that carries the mutation
- Sexual dimorphism
Differences between the phenotypes of males and females of the same species.
- Sexual intercourse
The physical activity of sex between two people.
- Sexual reproduction
A type of reproduction that involves a complex life cycle in which a gamete with a single set of chromosomes combines with another to produce an organism composed of cells with two sets of chromosomes.
- Short bones
Bones that are as wide as they are long. Their primary function is to provide support and stability with little to no movement.
- Single nucleotide polymorphism
A substitution of a single nucleotide that occurs at a specific position in the genome, where each variation is present at a level of 0.5% from person to person in the population.
- Sinoatrial node
A small body of specialized muscle tissue in the wall of the right atrium of the heart that acts as a pacemaker by producing a contractile signal at regular intervals.
- Sinus rhythm
The normal, rhythmical beating of the heart.
- Sister chromatids
Two identical copies formed by the DNA replication of a chromosome, with both copies joined together by a common centromere.
- Skeletal muscle
Voluntary, striated muscle that is attached to bones of the skeleton and helps the body move.
- Skeletal system
The body system composed of bones and cartilage and performs the following critical functions for the human body: supports the body. The skeletal system facilitates movement, protects internal organs, and produces blood cells.
- Skin
The major organ of the integumentary system that covers and protects the body and helps maintain homeostasis, for example, by regulating body temperature.
- Skull
The part of the human skeleton that provides a bony framework for the head and includes bones of the cranium and face.
- Sleep apnea
A disorder characterized by pauses in breathing during sleep, usually because of physical blockage of air flow.
- Sliding filament theory
A theory that explains muscle contraction by the sliding of myosin filaments over actin filaments within muscle fibers.
- Slow-twitch muscle fibers
A type of skeletal muscle cell that is mainly responsible for aerobic activities such as long-distance running.
- Small intestine
A long, narrow, tube-like organ of the digestive system where most chemical digestion of food and virtually all absorption of nutrients take place.
- Smell
A chemoreception (ability to detect the presence of certain chemicals) that, through the sensory olfactory system, forms the perception of smell.
- Smooth endoplasmic reticulum
An organelle found in eukaryotic cells with the function of making cellular products such as hormones and lipids. The smooth endoplasmic reticulum is a part of the endoplasmic reticulum that does not have attached ribosomes.
- Smooth muscle
An involuntary, nonstriated muscle that is found in the walls of internal organs such as the stomach.
- Sodium-potassium pump
A solute pump that pumps potassium into cells while pumping sodium out of cells, both against their concentration gradients. This pumping is active and occurs at the ratio of 2 potassium for every 3 calcium.
- Solution
A mixture of two or more substances that has the same composition throughout.
- Somatic mutation
Mutations acquired by a cell that can be passed to future cells arising from the mutated cell in the course of cell division.
- Somatic nervous system
A division of the peripheral nervous system that controls voluntary activities.
- Somatostatin
An endocrine hormone that inhibits the production of growth hormone by the pituitary and the secretion of insulin and glucagon by the pancreas, in addition to other functions.
- Special senses
A sense, such as vision or hearing, that has special sense organs that gather sensory information and change it into nerve impulses.
- Species
A population of similar organisms able to breed with one another.
- Sperm
The male reproductive cell.
- Spermatogenesis
The production or development of mature spermatozoa.
- Spermatogonium
A diploid stem cell in a testis that undergoes mitosis to begin the process of spermatogenesis.
- Sphincter
A ring of muscles that can contract to close off an opening between structures, such as between the esophagus and stomach.
- Spinal cavity
A long, narrow body cavity inside the vertebral column that runs the length of the trunk and contains the spinal cord.
- Spinal cord
A thin, tubular bundle of central nervous system tissue that extends from the brainstem down the back to the pelvis and connects the brain with the peripheral nervous system.
- Spleen
A secondary organ of the lymphatic system where blood and lymph are filtered.
- Spongy bone tissue
A light-weight, porous inner layer of bone that contains bone marrow.
- Squamous cell carcinoma
A common type of skin cancer that affects squamous cells in the epidermis and rarely metastasizes.
- Stable angina
A chest pain or discomfort that most often occurs with activity or emotional stress. Angina is due to poor blood flow through the blood vessels in the heart.
- Starch
A stored form of glucose used by plants.
- Stem cell
An undifferentiated cell that can develop into specialized types of cells.
- Stenosis
The abnormal narrowing of a passage in the body.
- Stereogram
A diagram or computer-generated image giving a three-dimensional representation of a solid object or surface.
- Sterilization
Surgical procedure that is generally irreversible, and makes it impossible for a woman to become pregnant or for a man to ejaculate viable, motile sperm.
- Steroid
A biologically active organic compound with four rings arranged in a specific molecular configuration. Steroids have two principal biological functions: as important components of cell membranes which alter membrane fluidity; and as signaling molecules.
- Steroid hormone
A type of endocrine hormone that is made of lipids and crosses the plasma membrane to bind with a receptor inside a target cell.
- Stimulant
A type of psychoactive drug that stimulates the brain and increases alertness and wakefulness.
- Stimulus
Something that triggers a behavior or other response.
- Stomach
A sac-like organ of the digestive system between the esophagus and small intestine in which both mechanical and chemical digestion take place.
- Stratum basale
The innermost (deepest) layer of the epidermis. Consists of a single layer of columnar or cuboidal basal cells.
- Stratum corneum
The outer layer of the skin (epidermis). It serves as the primary barrier between the body and the environment.
- Stratum granulosum
A thin layer of cells in the epidermis. Keratinocytes migrating from the underlying stratum spinosum become known as granular cells in this layer. Function is to help to form a waterproof barrier that functions to prevent fluid loss from the body.
- Stratum lucidum
a thin, clear layer of dead skin cells in the epidermis named for its translucent appearance under a microscope. It is readily visible by light microscopy only in areas of thick skin, which are found on the palms of the hands and the soles of the feet.
- Stratum spinosum
A layer of the epidermis found between the stratum granulosum and stratum basale. The main function of the stratum spinosum is to allow keratinocytes (cells that produce keratin) to mature.
- Stress urinary incontinence
The unintentional loss of urine. Stress incontinence happens when physical movement or activity — such as coughing, laughing, sneezing, running or heavy lifting — puts pressure (stress) on your bladder, causing you to leak urine.
- Stroke
A cerebrovascular accident in which a broken artery or blood clot results in lack of blood flow to part of the brain, causing death of brain cells.
- Sublingual gland
A salivary gland that is located under the floor of the mouth, close to the midline.
- Submandibular gland
A salivary gland that is located deep under the mandible (jawbone).
- Submucosa (digestive tract)
The layer of dense, irregular connective tissue or loose connective tissue that supports the mucosa, as well as joins the mucosa to the bulk of underlying smooth muscle (fibers that run circularly within a layer of longitudinal muscle).
- Substrate
A specific reactant in a chemical reaction which works with a specific enzyme.
- Sugar
The generic name for sweet-tasting, soluble carbohydrates, many of which are used in food. The various types of sugar are derived from different sources. Simple sugars are called monosaccharides and include glucose, fructose, and galactose.
- Sunburn
The reddening of the skin that occurs when the outer layer of the skin is damaged by UV light from the sun or tanning lamps.
- Surface area
The measure of how much exposed area a solid object has, expressed in square units.
- Surfactant
A mixture of lipids and proteins which is secreted by the epithelial type II cells into the alveolar space. Its main function is to reduce the surface tension at the air/liquid interface in the lung.
- Sutural bones
Accessory bones which occur within the skull. They get a different name, derivative from the suture or sutures they are in contact with or with the center of ossification or fontanel where they originate.
- Sweat
Salty fluid secreted into ducts by sweat glands in the dermis that excretes wastes and helps cool the body; also called perspiration.
- Sweat gland
An exocrine gland in the dermis of the skin that produces the salty fluid called sweat through a duct to the skin surface.
- Symbiotic
Any type of a close and long-term biological interaction between two different biological organisms.
- Sympathetic division
The division of the autonomic nervous system that controls the fight-or-flight response.
- Synapse
The place where the axon terminal of a neuron transmits a chemical or electrical signal to another cell.
- Synaptic cleft
A space that separates two neurons. It forms a junction between two or more neurons and helps nerve impulse pass from one neuron to the other.
- Synaptic vesicles
These membrane-bound organelles store various neurotransmitters that are released at the synapse. The release is regulated by a voltage-dependent calcium channel. Vesicles are essential for propagating nerve impulses between neurons and are constantly recreated by the cell.
- Synovial joint
A movable joint in which a fluid-filled synovial cavity separates bones at the joint.
- Systemic circuit
The part of the cardiovascular system that carries blood between the heart and body.
- Systemic lupus erythematosus
A chronic disease that causes inflammation in connective tissues, such as cartilage and the lining of blood vessels, which provide strength and flexibility to structures throughout the body.
- Systole
The part of a heartbeat in which the atria relax and fill with blood from the lungs and body, while the ventricles contract and pump blood out of the heart.
- T cell
A type of lymphocyte that kills infected or cancerous cells (killer T cell) or helps regulate the immune response (helper T cell).
- T3
An endocrine hormone secreted by the thyroid gland that increases the rate of metabolism in cells throughout the body.
- T4
An endocrine hormone secreted by the thyroid gland that increases the rate of metabolism in cells throughout the body.
- Target cell
A type of cell on which a particular hormone has an effect because it has receptor molecules for the hormone.
- Taste
The perception produced or stimulated when a substance in the mouth reacts chemically with taste receptor cells located on taste buds in the oral cavity, mostly on the tongue.
- Taste bud
A small structure on the tongue containing chemoreceptor cells that sense chemicals in food.
- Taste pore
Small openings in the tongue epithelium, which allow parts of the food dissolved in saliva come into contact with taste receptors.
- TATA box
A DNA sequence that indicates where a genetic sequence can be read and decoded. It is a type of promoter sequence, which specifies to other molecules where transcription begins.
- Taxonomy
The science of classifying organisms.
- Teeth
A hard structure, embedded in the jaws of the mouth, that functions in chewing. Made of a dentin and covered in enamel, the hardest tissue in the body.
- Telophase
The final phase of cell division, between anaphase and interphase, in which the chromatids or chromosomes move to opposite ends of the cell and two nuclei are formed.
- Temporal lobe
Part of each hemisphere of the cerebrum that is involved in functions such as hearing, memories, and sensory integration.
- Tendinitis
Inflammation of a tendon when it is over-extended or worked too hard without rest.
- Tendon
Dense fibrous connective tissue that attaches skeletal muscle to bones.
- Testes (singular: testis)
Two male reproductive organs that produce sperm and secrete testosterone; male gonad.
- Testicular cancer
Cancer of the testes, which is more common in younger men.
- Testosterone
The male sex hormone secreted mainly by the testes.
- Thalamus
The inner part of the brain that is a major hub for nerve impulses traveling back and forth between the cerebrum and spinal cord.
- Theory
An explanation of an aspect of the natural world that can be repeatedly tested and verified in accordance with the scientific method.
- Thermoreceptor
A type of sensory receptor that senses temperature.
- Thoracic cavity
A body cavity in the chest that holds the lungs and heart.
- Thoracic vertebrae
each of the twelve bones of the backbone to which the ribs are attached.
- Threshold
The critical level to which a membrane potential must be depolarized to initiate an action potential.
- Thrifty gene hypothesis
A hypothesis which suggests that the carriers of the 'thrifty genes' survive because they deposit fat between famines. The implication of this is that the primary factor causing famine mortality is running out of energy reserves—that is, starvation, and that fatter people run out of reserves more slowly.
- Thrombocyte
Another term for platelet; a small colorless disk-shaped cell fragment without a nucleus, found in large numbers in blood and involved in clotting.
- Thymus
An organ of the lymphatic system where lymphocytes called T cells mature.
- Thymus gland
An organ of the lymphatic system where lymphocytes called T cells mature.
- Thyroglobulin
A glycoprotein produced by the follicular cells of the thyroid and used entirely within the thyroid gland.
- Thyroid gland
A large endocrine gland in the neck whose hormones control the rate of cellular metabolism and help maintain calcium homeostasis.
- Thyroid stimulating hormone (TSH)
A pituitary hormone that stimulates the thyroid gland to produce thyroxine, and then triiodothyronine which stimulates the metabolism of almost every tissue in the body.
- Thyrotropin releasing hormone (TRH)
A hormone, produced by neurons in the hypothalamus, that stimulates the release of thyroid-stimulating hormone and prolactin from the anterior pituitary.
- Thyroxine
An endocrine hormone secreted by the thyroid gland that increases the rate of metabolism in cells throughout the body.
- Tissue
A cellular organizational level between cells and a complete organ. A tissue is an ensemble of similar cells and their extracellular matrix from the same origin that together carry out a specific function. Organs are then formed by the functional grouping together of multiple tissues.
- Tongue
The fleshy muscular organ in the mouth used for tasting, licking, swallowing, and articulating speech.
- Tonsil
An organ of the lymphatic system where lymphocytes called T cells mature.
- Touch
The ability to sense pressure, vibration, temperature, pain, and other tactile stimuli.
- Toxoplasmosis
A disease that results from infection with the Toxoplasma gondii parasite, one of the world's most common parasites. Infection usually occurs by eating undercooked contaminated meat, exposure from infected cat feces, or mother-to-child transmission during pregnancy.
- Trachea
A tubular organ of the respiratory system that carries air between the larynx and bronchi; also called windpipe.
- Transcription
The process by which DNA is copied (transcribed) to mRNA in order transfer the information needed for protein synthesis.
- Transfer RNA (tRNA)
A small RNA molecule that participates in protein synthesis. Each tRNA molecule has two important areas: an anticodon and a region for attaching a specific amino acid.
- Transgenic crops
Plants used in agriculture, the DNA of which has been modified using genetic engineering methods. In most cases, the aim is to introduce a new trait to the plant which does not occur naturally in the species.
- Translation
The process in which mRNA along with transfer RNA (tRNA) and ribosomes work together to produce polypeptides.
- Transport protein
A membrane protein involved in the movement of ions, small molecules, or macromolecules, such as another protein, across a biological membrane.
- Traumatic brain injury
Sudden damage to the brain caused by a blow or jolt to the head. Common causes include car or motorcycle crashes, falls, sports injuries, and assaults. Injuries can range from mild concussions to severe permanent brain damage.
- Tricuspid atrioventricular valve
A valve in the heart which forms the boundary between the right ventricle and the right atrium. Deoxygenated blood enters the right side of the heart via the inferior and superior vena cava. It contains three flap-like cusps that, when closed, keep blood from regressing back into the right atrium.
- Triglycerides
A type of lipid consisting of a glycerol and three fatty acids. Triglycerides are a form of energy storage used in animals (fats) and plants (oils).
- Triiodothyronine
An endocrine hormone secreted by the thyroid gland that increases the rate of metabolism in cells throughout the body.
- Trimester
One of three, approximately three-month periods into which a pregnancy is divided.
- Trypsin
A digestive enzyme that breaks down proteins in the small intestine. It is secreted by the pancreas in an inactive form, trypsinogen.
- Tubal ligation
Surgical sterilization procedure in females in which the Fallopian tubes are blocked so sperm cannot reach and fertilize an egg.
- Tumor
A mass of tissue that's formed by an accumulation of abnormal cells.
- Tumor suppressor genes
Normal genes that slow down cell division, repair DNA mistakes, or tell cells when to die (a process known as apoptosis or programmed cell death). When tumor suppressor genes don't work properly, cells can grow out of control, which can lead to cancer.
- Tunica albuginea
The middle layer of the tunica of the testes. It is a dense layer of fibrous tissue that encases the testis. It also extends into the testis, creating the septa between lobules.
- Tunica externa
The outermost layer of a blood vessel, surrounding the tunica media. It is mainly composed of collagen and, in arteries, is supported by external elastic lamina.
- Tunica intima
The innermost layer of an artery or vein. It is made up of one layer of endothelial cells and is supported by an internal elastic lamina. The endothelial cells are in direct contact with the blood flow.
- Tunica media
The middle layer of an artery or vein. It lies between the tunica intima on the inside and the tunica externa on the outside. It consists of connective tissues, elastic fibers and smooth muscle.
- Tunica vaginalis
The outmost layer of the tunica of the testes. It actually consists of two layers of tissue separated by a thin layer of serous fluid which reduces friction between the testes and the scrotum.
- Tunica vasculosa
The innermost layer of the tunica of the testes. It consists of connective tissue and contains arteries and veins that carry blood to and from the testis.
- Type 1 diabetes
An autoimmune disorder in which the immune system destroys insulin-secreting beta cells in the pancreas, leading to loss of glucose control and high levels of blood glucose.
- Type 2 diabetes
A multifactorial disorder in which a combination of insulin resistance and impaired insulin production lead to loss of glucose control and high levels of blood glucose.
- Type 2 diabetes
A multifactorial disorder in which a combination of insulin resistance and impaired insulin production lead to loss of glucose control and high levels of blood glucose.
- Typology
A classification according to general type, especially in archaeology, psychology, or the social sciences.
- Ulcerative colitis
An inflammatory bowel disease that causes ulcers (sores) in the colon and rectum.
- Unsaturated fatty acid
A fat or fatty acid in which there is at least one double bond within the fatty acid chain. A fatty acid chain is monounsaturated if it contains one double bond, and polyunsaturated if it contains more than one double bond.
- Unstable angina
A condition in which your heart doesn't get enough blood flow and oxygen. It may lead to a heart attack. Angina is a type of chest discomfort caused by poor blood flow through the blood vessels (coronary vessels) of the heart muscle (myocardium).
- Upper GI tract
The part of the gastrointestinal tract that includes the mouth, pharynx, esophagus, and stomach.
- Upper respiratory tract
Refers to following airway structures: nasal cavities and passages (sinuses), pharynx, tonsils, and larynx (voice box).
- Urea
Waste product of protein catabolism that is mainly filtered from blood in the kidneys and excreted in urine.
- Ureter
A muscular, tube-like organ of the urinary system that moves urine by peristalsis from a kidney to the bladder.
- Urethra
A tube-like organ of the urinary system that carries urine out of the body from the bladder and, in males, also carries semen out of the body.
- Urge urinary incontinence
When you have a strong, sudden need to urinate that is difficult to delay. The bladder then squeezes, or spasms, and you lose urine.
- Uric acid
A waste product of nucleic acid catabolism that is mainly filtered from blood by the kidneys and excreted in urine.
- Urinalysis
An analysis of urine by physical, chemical, and microscopical means to test for the presence of disease, drugs, etc.
- Urinary bladder
A sac-like organ that stores urine until it is excreted from the body.
- Urinary incontinence
A common chronic problem of uncontrolled leakage of urine.
- Urinary system (also known as the renal system)
The body system that produces, stores and eliminates urine, the fluid waste excreted by the kidneys. The kidneys make urine by filtering wastes and extra water from blood. Urine travels from the kidneys through two thin tubes called ureters and fills the bladder.
- Urination
The process in which urine leaves the body through the external urethral orifice.
- Urine
A liquid waste product of the body that is formed by the kidneys and excreted by the other organs of the urinary system.
- Uterine cycle
The events of the menstrual cycle that occur in the uterus, including menses and the buildup of the endometrium.
- Uterus
The female reproductive organ in which first an embryo and then a fetus grows and develops until birth.
- UV light
A form of electromagnetic radiation with wavelength shorter than that of visible light but longer than X-rays. UV radiation is present in sunlight, and constitutes about 10% of the total electromagnetic radiation output from the sun.
- Vaccine
A substance used to stimulate the production of antibodies and provide immunity against one or several diseases, prepared from the causative agent of a disease, its products, or a synthetic substitute, treated to act as an antigen without inducing the disease.
- Vacuole
A membrane-bound organelle which is present in all plant and fungal cells and some protist, animal and bacterial cells. It's function is storage of substances and to maintain the rigidity of plant cells.
- Vagina
The female reproductive organ that receives sperm during sexual intercourse and provides a passageway for a baby to leave the mother’s body during birth.
- Vaginitis
An inflammation of the vagina usually caused by an infection with microbes.
- Vas deferens
One of a pair of thin tubes that transports sperm from an epididymis to an ejaculatory duct during ejaculation; also called sperm duct.
- Vasectomy
Surgical sterilization procedure in males in which the vas deferens are blocked so sperm cannot be ejaculated.
- Vasoconstriction
A narrowing of blood vessels so less blood can flow through them.
- Vasodilation
The widening of blood vessels. It results from relaxation of smooth muscle cells within the vessel walls, in particular in the large veins, large arteries, and smaller arterioles. The process is the opposite of vasoconstriction, which is the narrowing of blood vessels.
- Vasopressin
A nonapeptide synthesized in the hypothalamus, also referred to as antidiuretic hormone (ADH) or arginine vasopressin (AVP). Science has known it to play essential roles in the control of the body's osmotic balance, blood pressure regulation, sodium homeostasis, and kidney functioning.
- Vector
A carrier genetically engineered to deliver a gene. Certain viruses are often used as vectors because they can deliver the new gene by infecting the cell.
- Vein
A type of blood vessel that carries blood toward the heart from the lungs or body.
- Vena cava
A large vein carrying deoxygenated blood into the heart. There are two in humans, the inferior vena cava (carrying blood from the lower body) and the superior vena cava (carrying blood from the head, arms, and upper body).
- Ventilation
The process of moving air into and out of the lungs; also called breathing.
- Ventral cavity
A major human body cavity at the anterior (front) of the trunk that contains such organs as the lungs, heart, stomach, intestines, and internal reproductive organs.
- Ventricle
One of two lower chambers of the heart that pumps blood out of the heart.
- Vertebrae
One of 33 small bones that make up the vertebral column.
- Vertebral column
A flexible column of vertebrae that connects the trunk to the skull and encloses the spinal cord; also called spine or backbone.
- Vesicle
A structure within a cell, consisting of lipid bilayer. Vesicles form naturally during the processes of secretion, uptake and transport of materials within the plasma membrane.
- Vesicle transport
A form of active transport in which substances cross the plasma membrane with the help of a vesicle.
- Villi (singular, villus)
A microscopic, finger-like projections in a mucous membrane that form a large surface area for absorption.
- Virus
A tiny, nonliving particle that contains nucleic acids but lacks other characteristics of living cells and may cause human disease.
- Vision
The ability to sense light and see; also called sight.
- Vitreous humor
The clear gel that fills the space between the lens and the retina of the eyeball of humans and other vertebrates. It is often referred to as the vitreous humor or simply "the vitreous".
- Vocal cords
Two folded pairs of membranes in the larynx (voice box) that vibrate when air that is exhaled passes through them, producing sound.
- Voice box (also known as the larynx)
The portion of the respiratory (breathing) tract containing the vocal cords which produce sound.
- Voluntary
Actions which take place according to the one's desire or are under control.
- Vomiting
The involuntary process of ejecting matter from the stomach through the mouth.
- Vulva
External female reproductive structures, including the clitoris, labia, and vaginal and urethral openings.
- White matter
A type of nervous tissue that consists mainly of the myelinated axons of neurons.
- X-linked genes
Genes causing a trait or disorder which are present on the X sex determining chromosome.
- X-linked trait
A trait where a gene is located on the X chromosome.
- Zona fasciculata
The middle layer of the adrenal cortex. It is the largest of the three zones, accounting for nearly 80% of the adrenal cortex.
- Zona glomerulosa
The outermost layer of the adrenal cortex. It lies immediately under the outer fibrous capsule that encloses the adrenal gland.
- Zona reticularis
The innermost layer of the adrenal cortex. It is directly adjacent to the medulla of the adrenal gland.
- Zygote
The union of the sperm cell and the egg cell. Also known as a fertilized ovum, the zygote begins as a single cell but divides rapidly in the days following fertilization. After this two-week period of cell division, the zygote eventually becomes an embryo.








![By Thebiologyprimer [CC0], from Wikimedia Commons Diagram shows the scientific cycle arranged in a circular formation: Observation, questions, hypothesis, experiment, analysis, conclusion and then returning to observation again.](http://humanbiology.pressbooks.tru.ca/wp-content/uploads/sites/6/2018/10/The_Scientific_Method_simple-300x280.png)
![Photo by Ministry of Information Photo Division Photographer [Public domain], via Wikimedia Commons A black and white photo of Alexander Fleming examining bacterial growth on a petri dish.](http://humanbiology.pressbooks.tru.ca/wp-content/uploads/sites/6/2018/10/Alexander-Flemming-300x251.jpg)

![Image by Francesco Redenti 1820-1876. (Wellcome Library Record no. 3120i) [Public domain], via Wikimedia Commons A black and white side-profile caricature of Girolamo Fracastoro wearing tradition middle-18th century attire.](http://humanbiology.pressbooks.tru.ca/wp-content/uploads/sites/6/2018/10/Redenti_-_Girolamo_Fracastoro-226x300.jpg)
![Graph by Power.corrupts [Public domain], from Wikimedia Commons](http://humanbiology.pressbooks.tru.ca/wp-content/uploads/sites/6/2018/10/1024px-Yearly_mortality_rates_1833-1858-300x185.png)
![Image by By Photo Credit:Content Providers(s): [Public domain], via Wikimedia A view through a microscope showing larger irregularly oval blue cells, and strings of smaller yellow round cells. The chains of small yellow cells are the Streptococcus pyogenes.](http://humanbiology.pressbooks.tru.ca/wp-content/uploads/sites/6/2018/10/Streptococcus_pyogenes_01_thumbnail.png)
![Image by Albert Edelfelt [Public domain], via Wikimedia Commons A painting showing Louis Pasteur sitting in his lab examining a substance in a bottle](http://humanbiology.pressbooks.tru.ca/wp-content/uploads/sites/6/2018/10/Tableau_Louis_Pasteur-246x300.jpg)





![Image by By James Howard McGregor [Public domain], via Wikimedia Commons A side profile view of an artists rendition of what the Piltdown Man may have looked like, had he been real.](http://humanbiology.pressbooks.tru.ca/wp-content/uploads/sites/6/2018/10/Piltdown-Man-300x285.jpg)
![Photo by Anrie [CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0)], from Wikimedia Commons A replica of the infamous Piltdown skull. The skull is encased in a glass sphere. The replica shows portions of the skull which were bone in white, and the portions of the skull which were inferred in black.](http://humanbiology.pressbooks.tru.ca/wp-content/uploads/sites/6/2018/10/Sterkfontein_Piltdown_man-300x294.jpg)




![Auguste Rodin [CC0] The Thinker (French: Le Penseur) is a bronze sculpture by Auguste Rodin, usually placed on a stone pedestal. The work shows a nude male figure of over life-size sitting on a rock with his chin resting on one hand as though deep in thought, often used as an image to represent philosophy.](http://humanbiology.pressbooks.tru.ca/wp-content/uploads/sites/6/2018/10/The-thinker-225x300.jpg)
![Joseph Elsbernd [CC BY-SA 2.0 (https://creativecommons.org/licenses/by-sa/2.0)] Shows the image through a microscope of human cheek cells. The cells are oval in shape and light blue, with a darker blue spot close to the centre. The light blue shows the cell membrane and cytoplasm and the darker blue shows the nucleus of the cell.](http://humanbiology.pressbooks.tru.ca/wp-content/uploads/sites/6/2018/10/Cheek-Cells-300x200.jpg)
![kaibara87 [CC BY 2.0 (https://creativecommons.org/licenses/by/2.0)] Shows an image through a microscope of onion cells. The cells are packed together and are rectangular in shape. Their cell walls and nuclei are stained a darker blue and the cytoplasm is whitish.](http://humanbiology.pressbooks.tru.ca/wp-content/uploads/sites/6/2018/10/Onion-Cells-300x200.jpg)







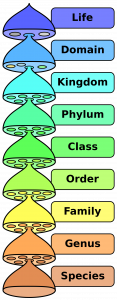
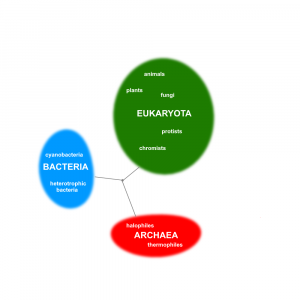
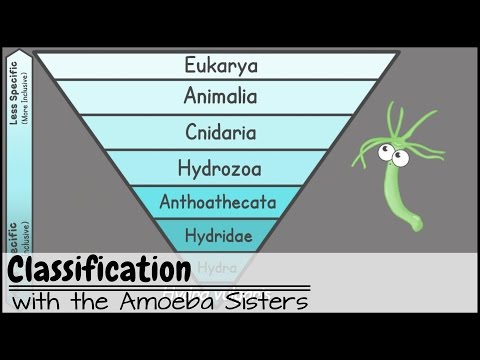

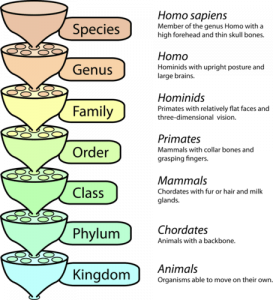











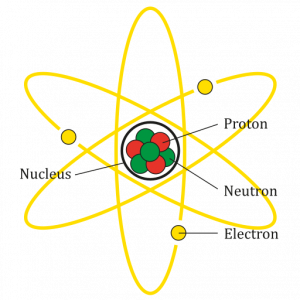







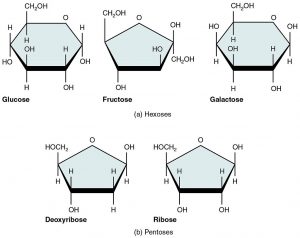

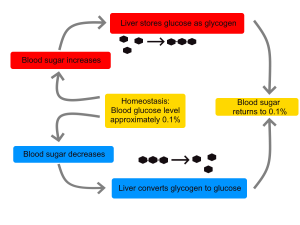


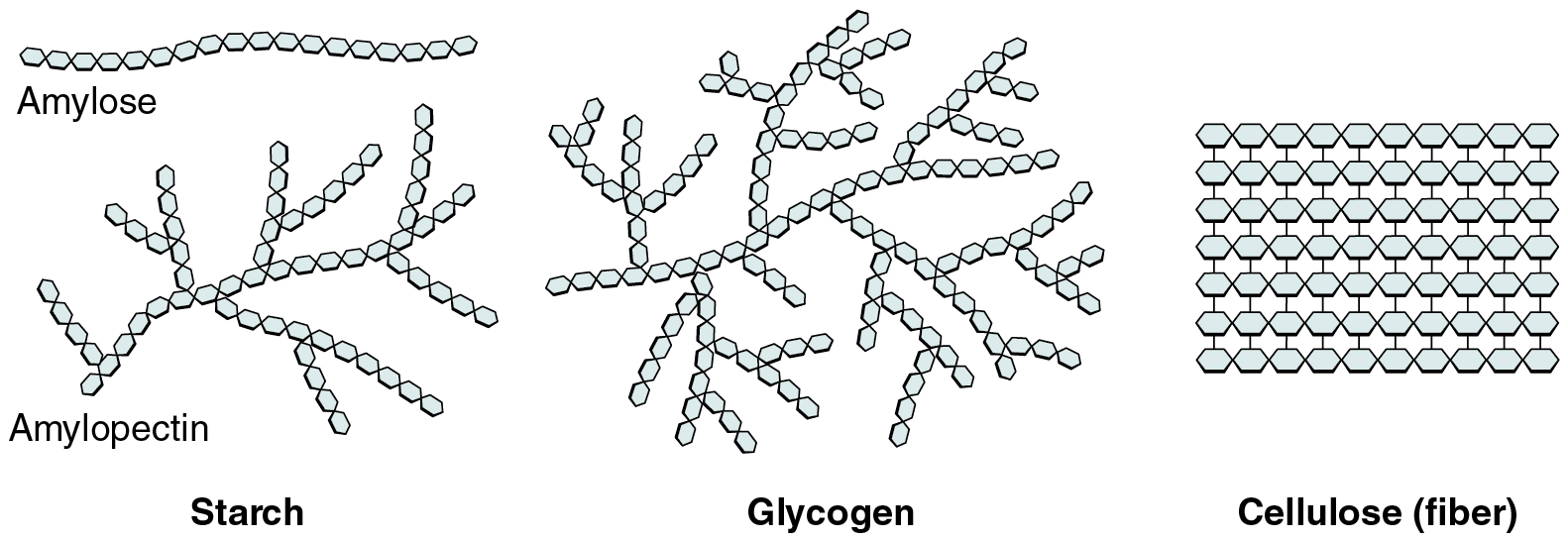






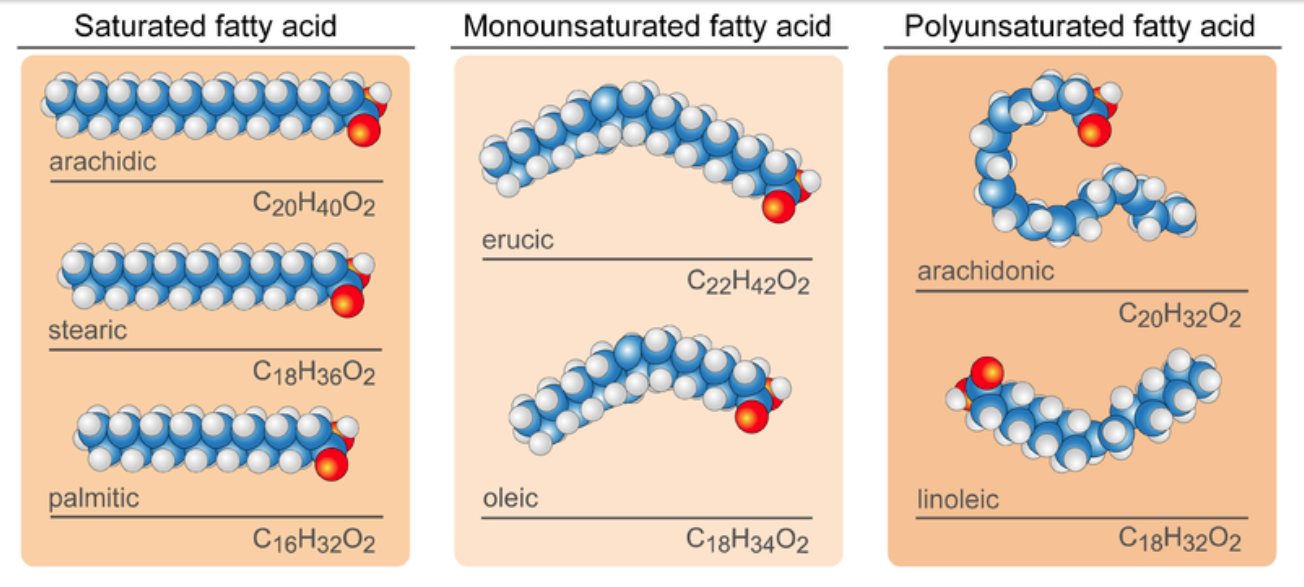
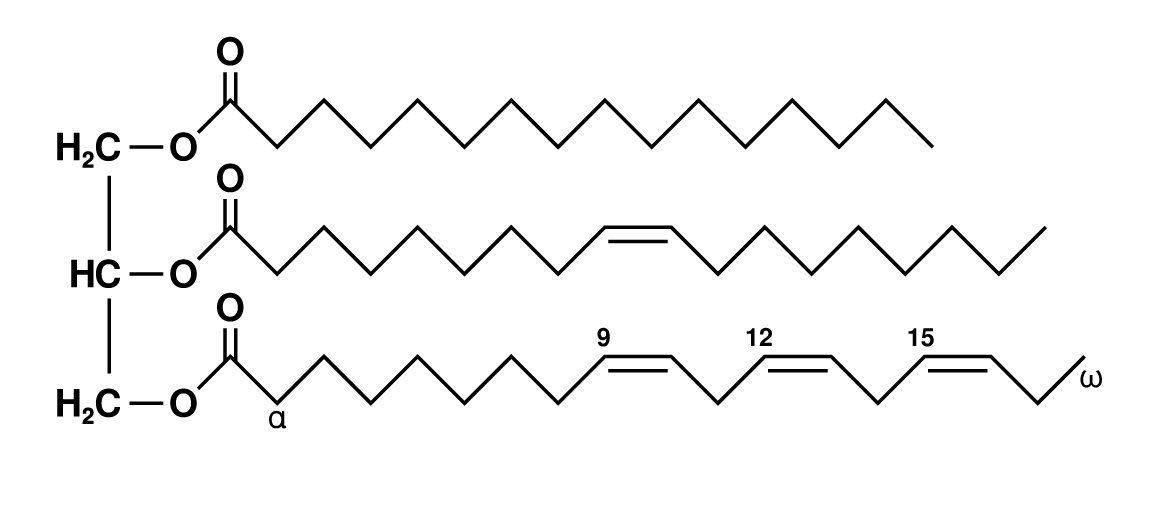
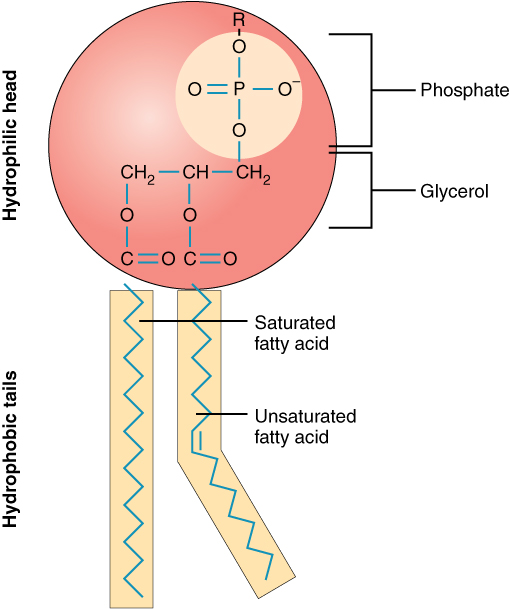
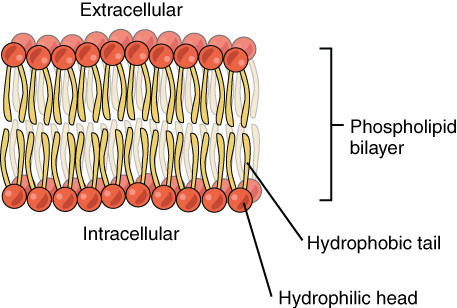
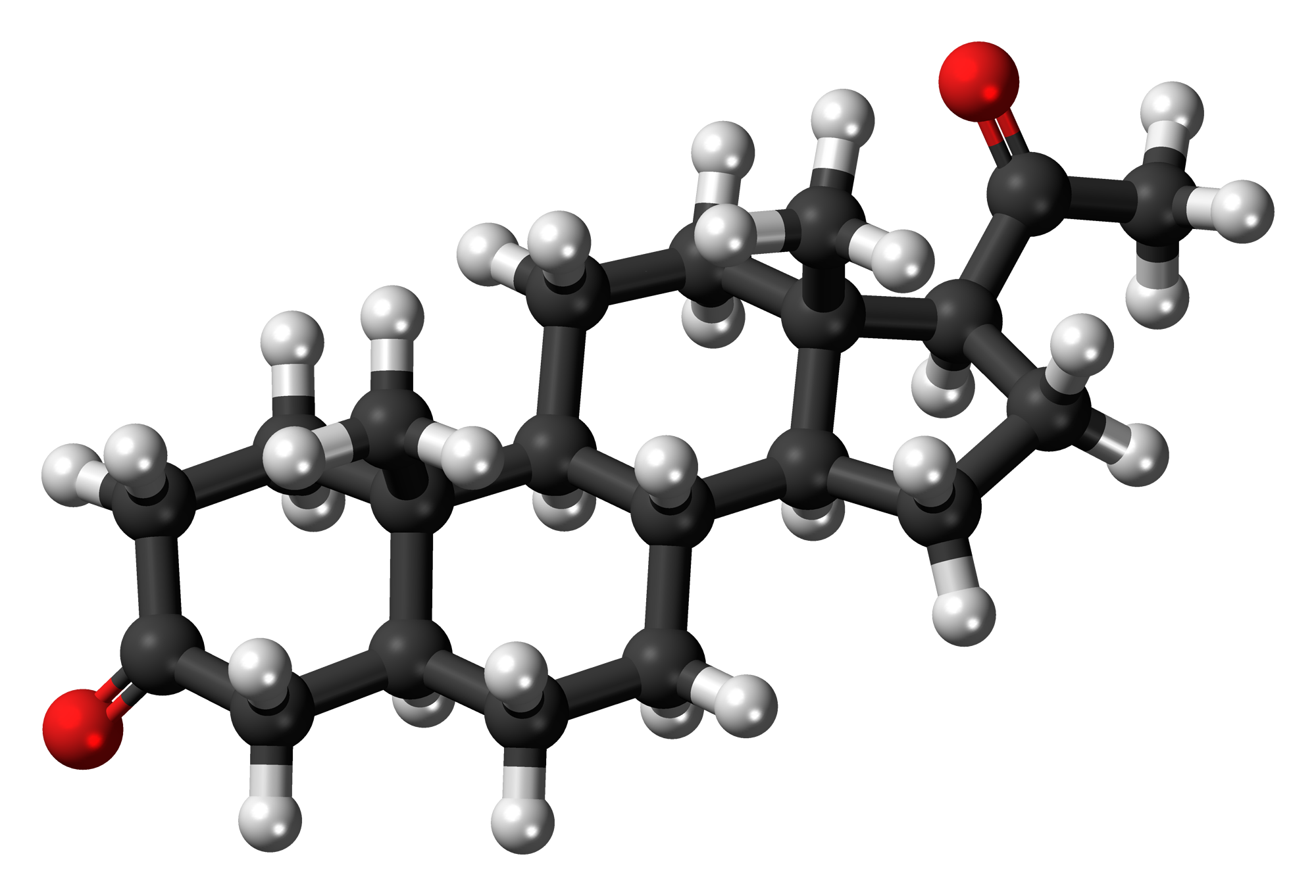




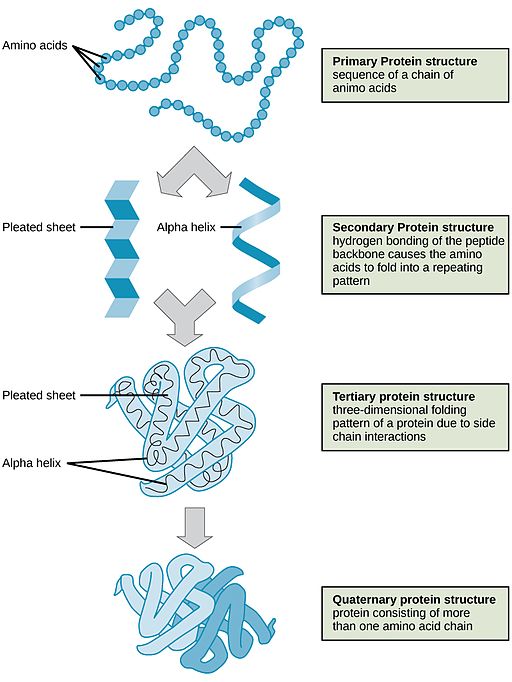
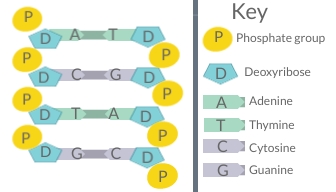
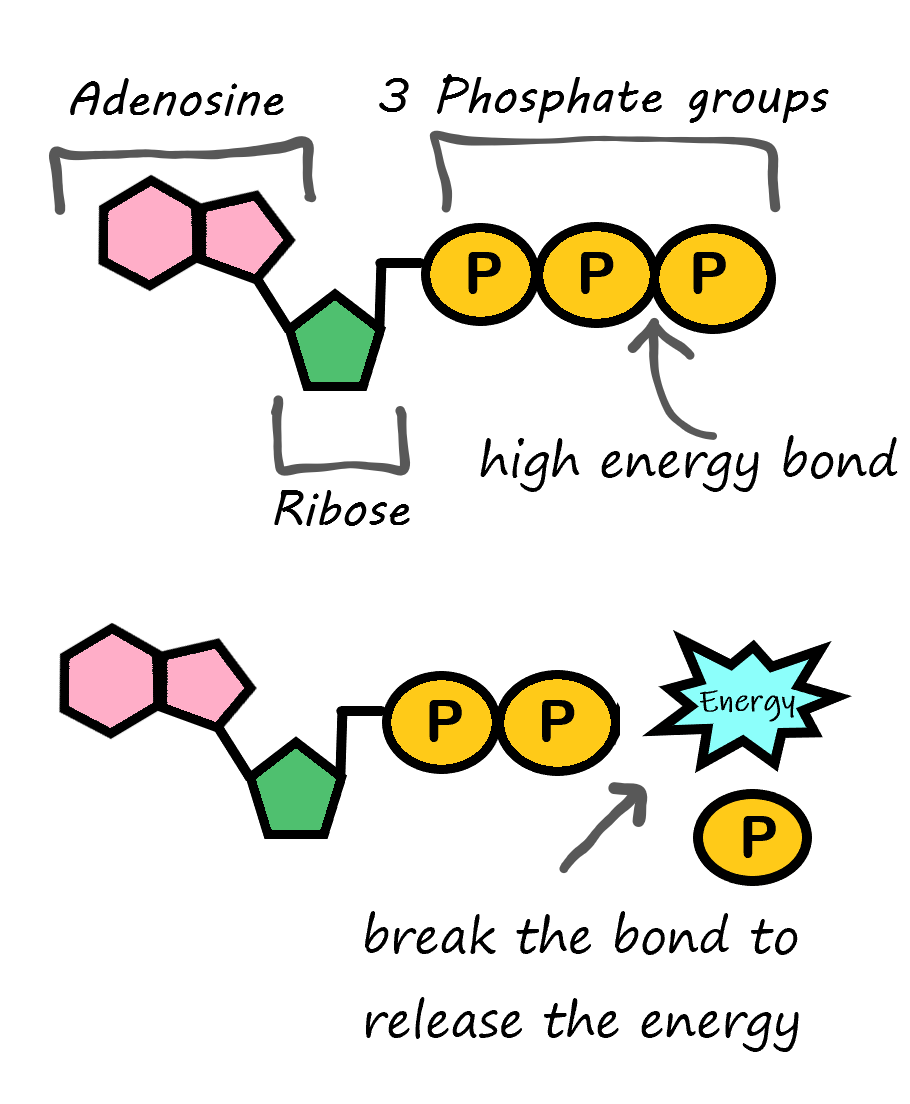









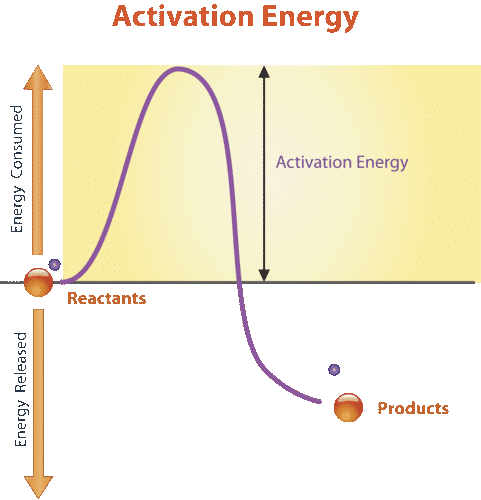



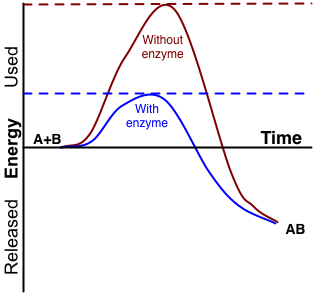









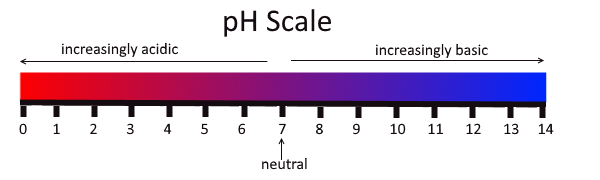





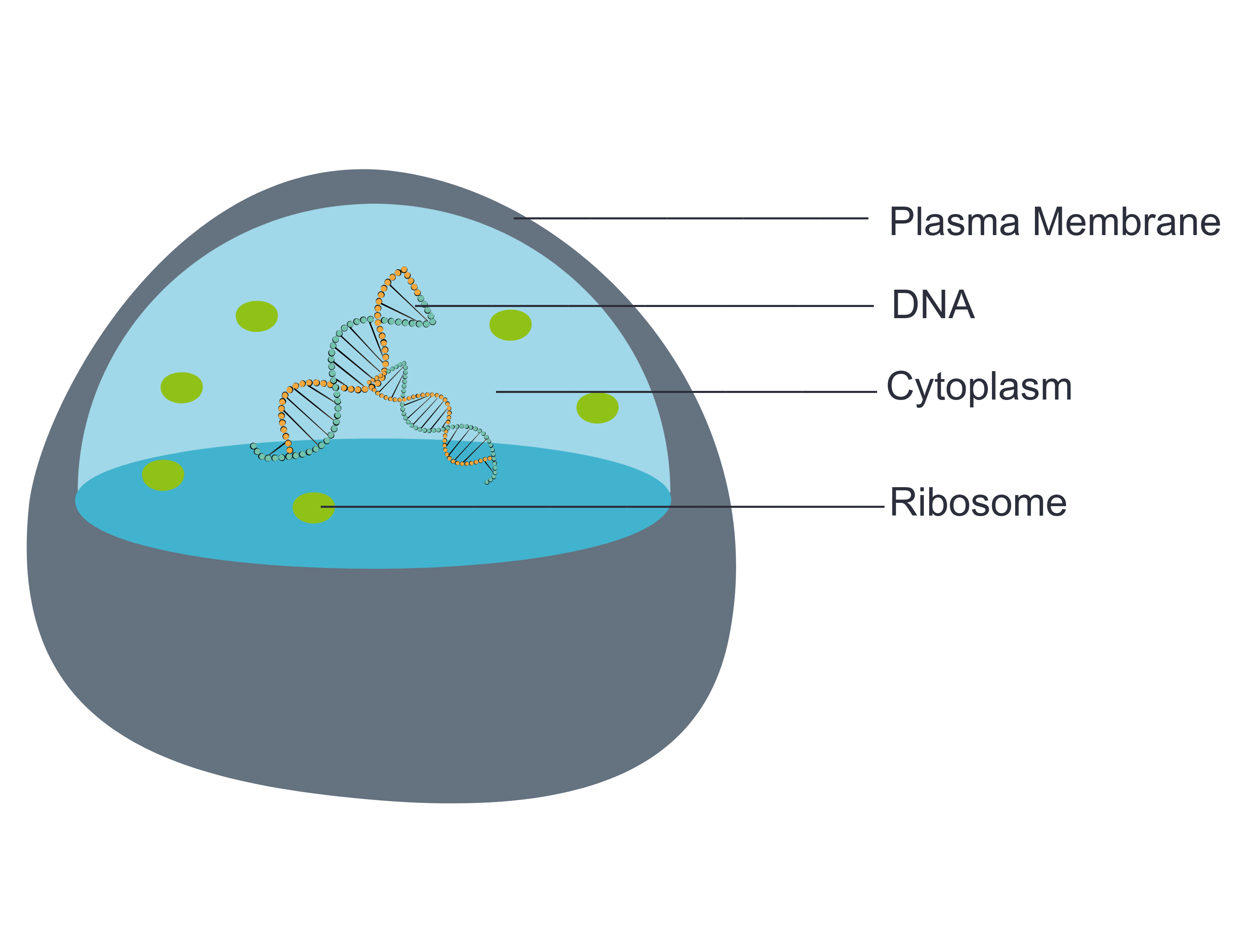

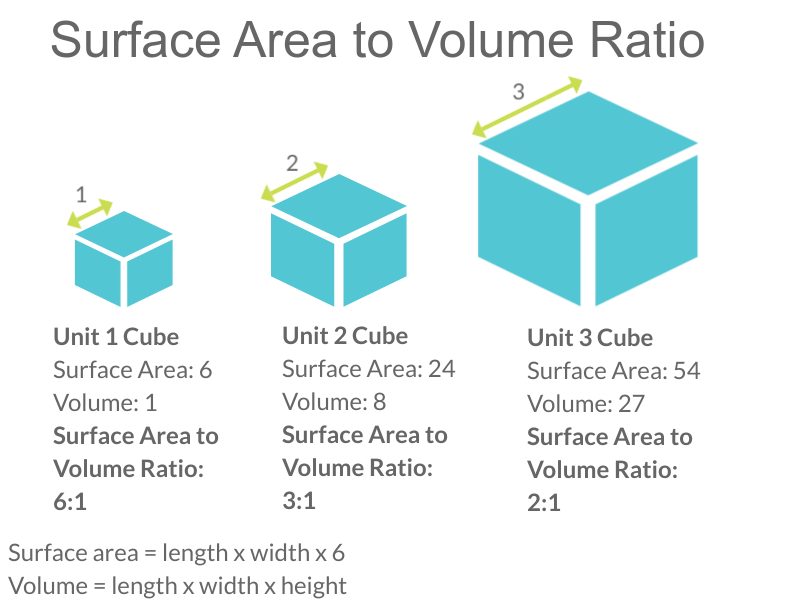
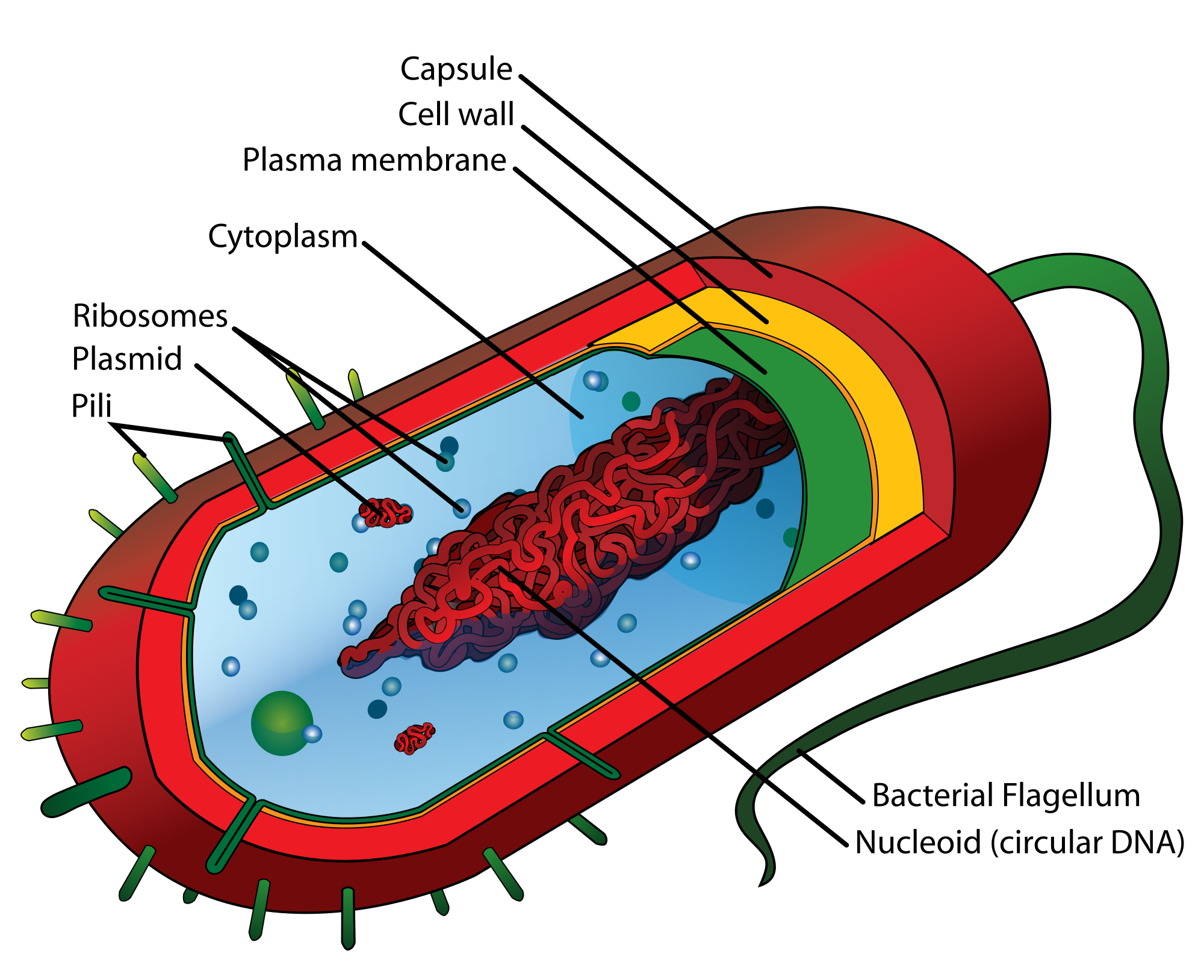
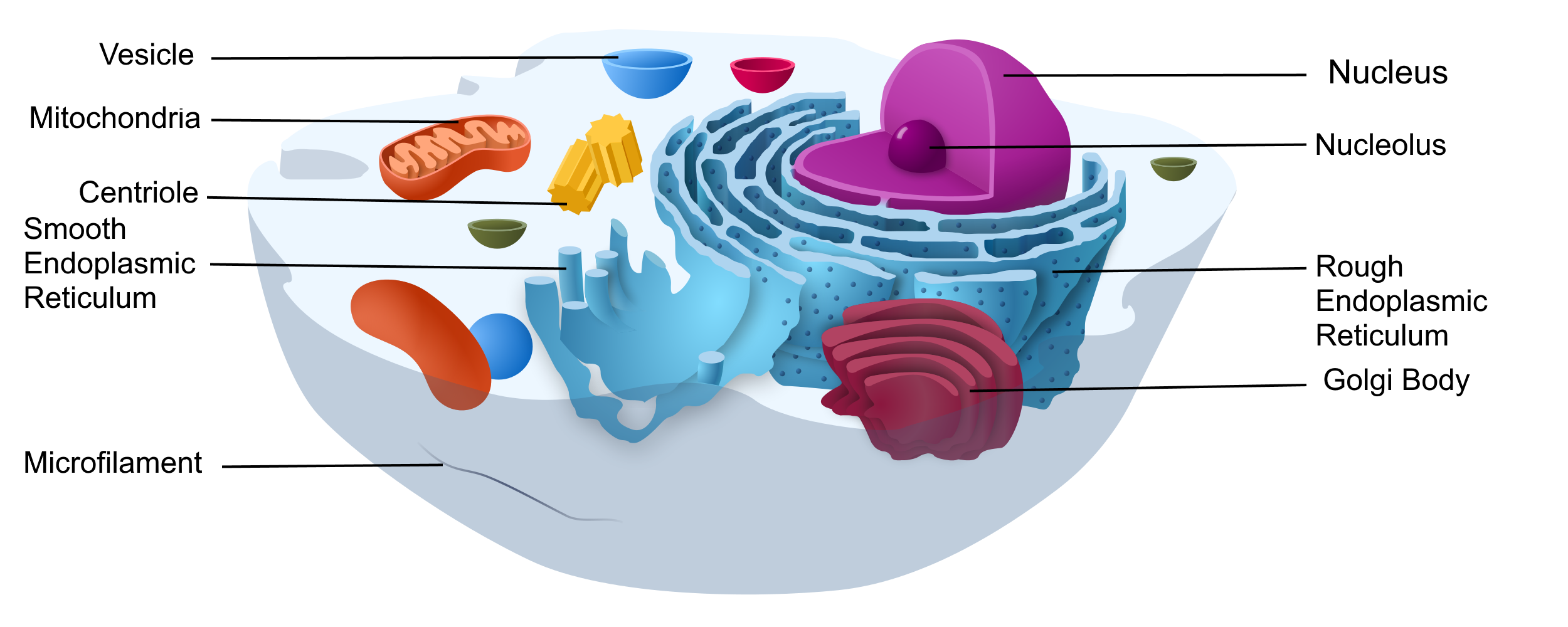




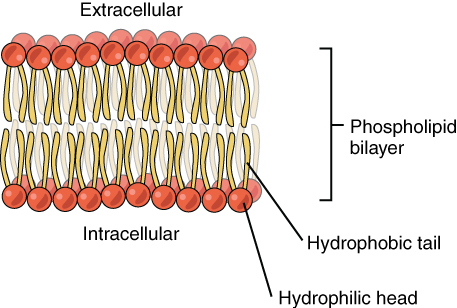
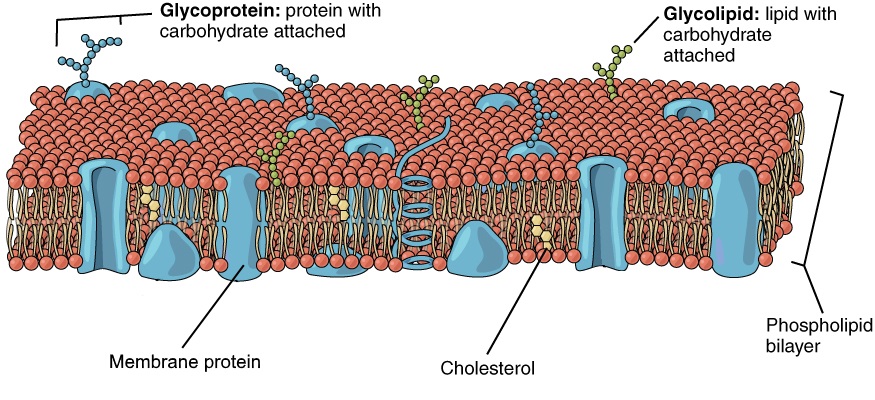

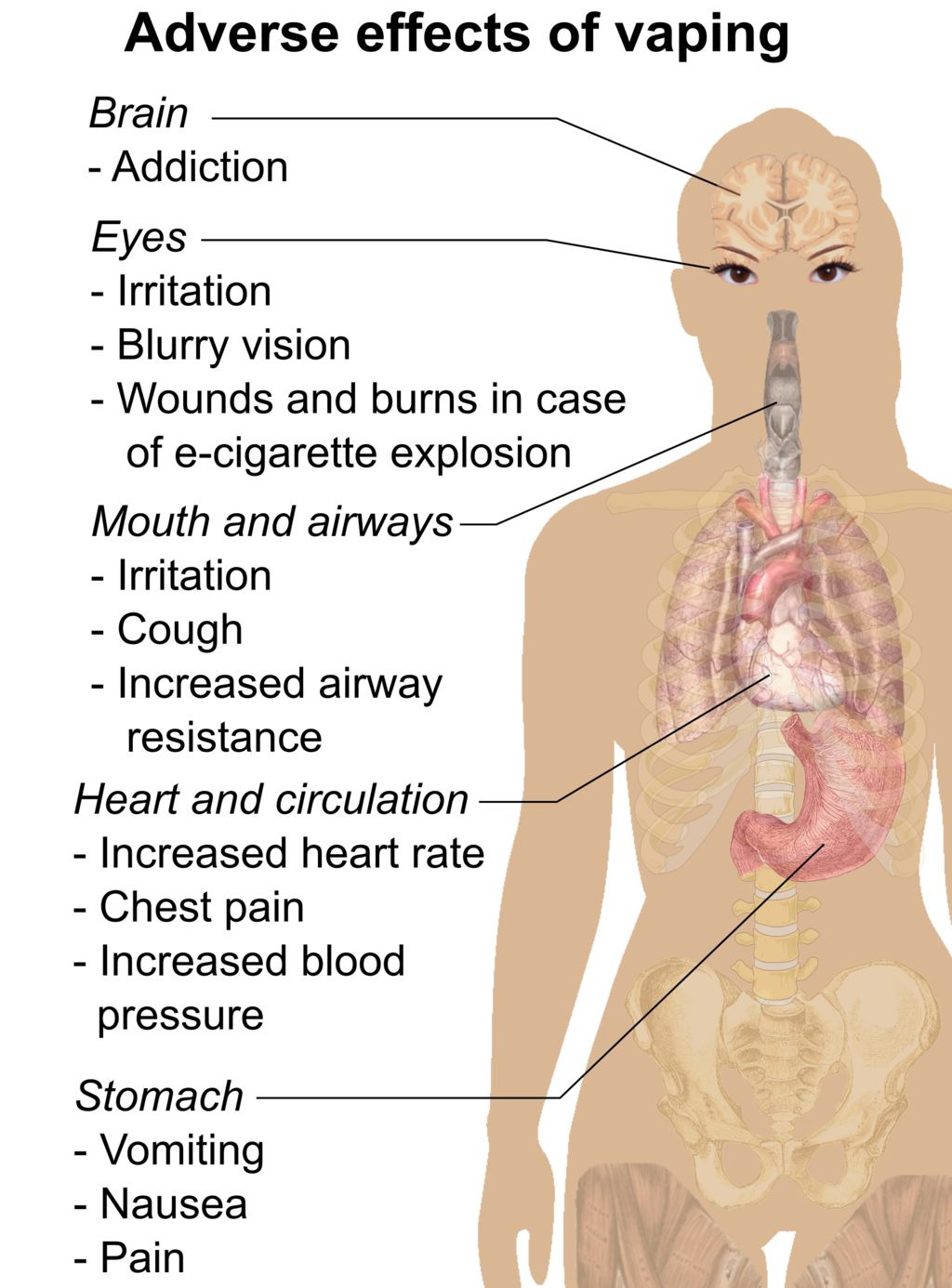

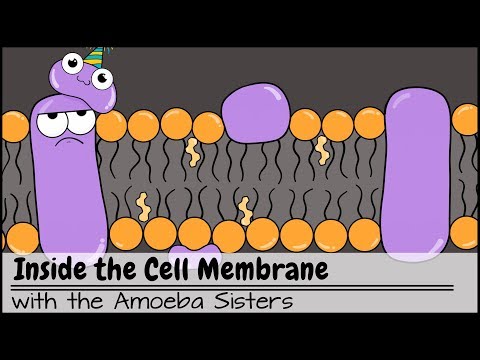
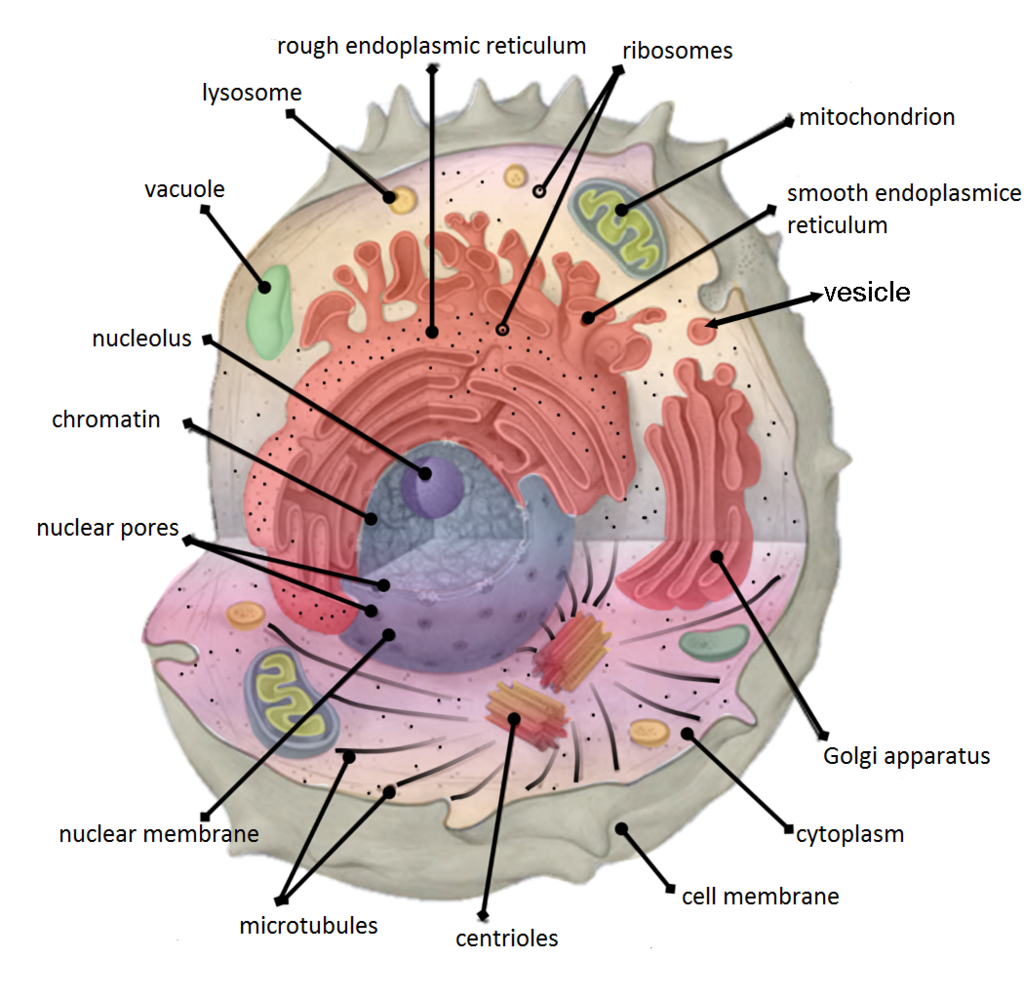

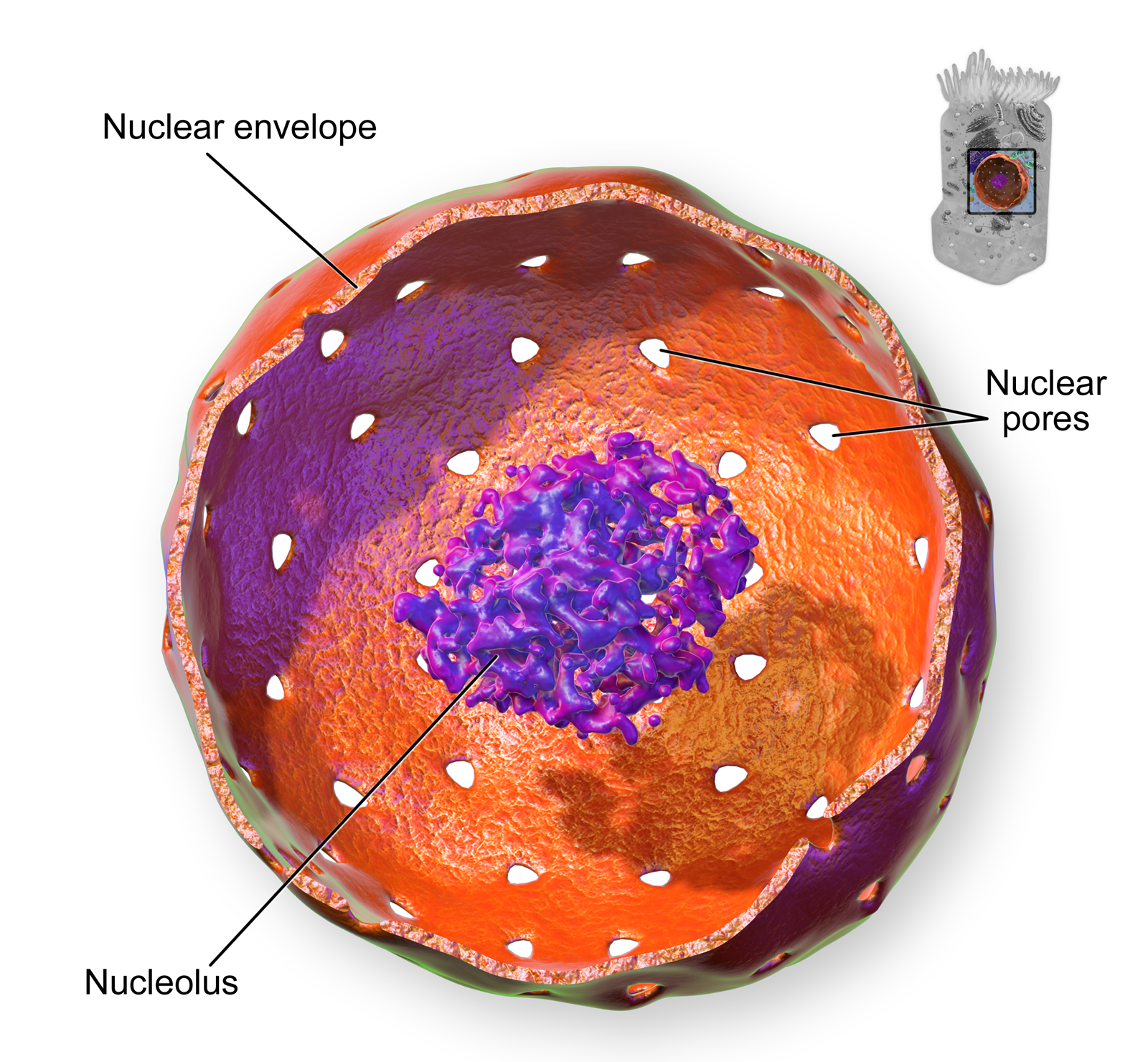
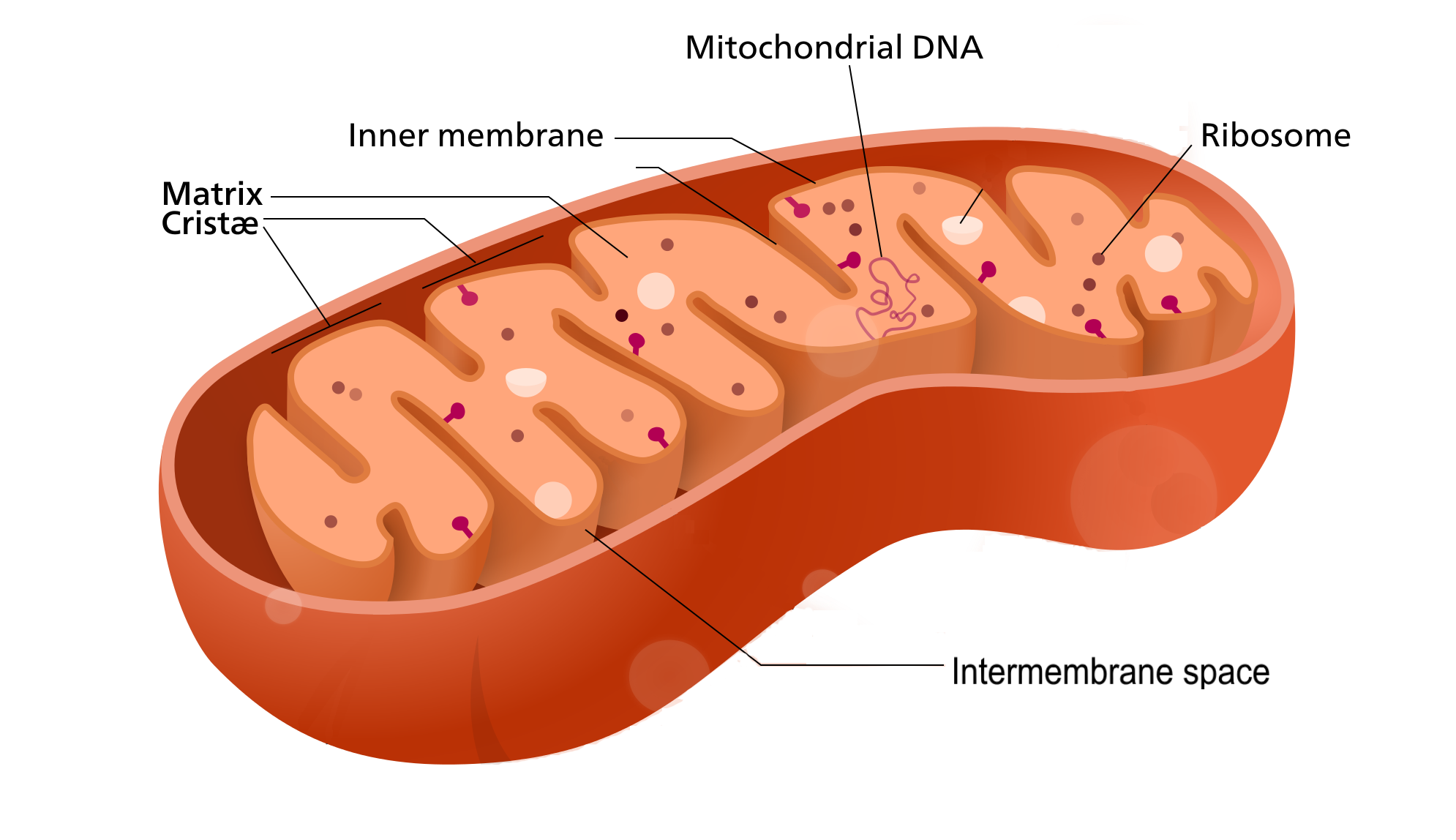
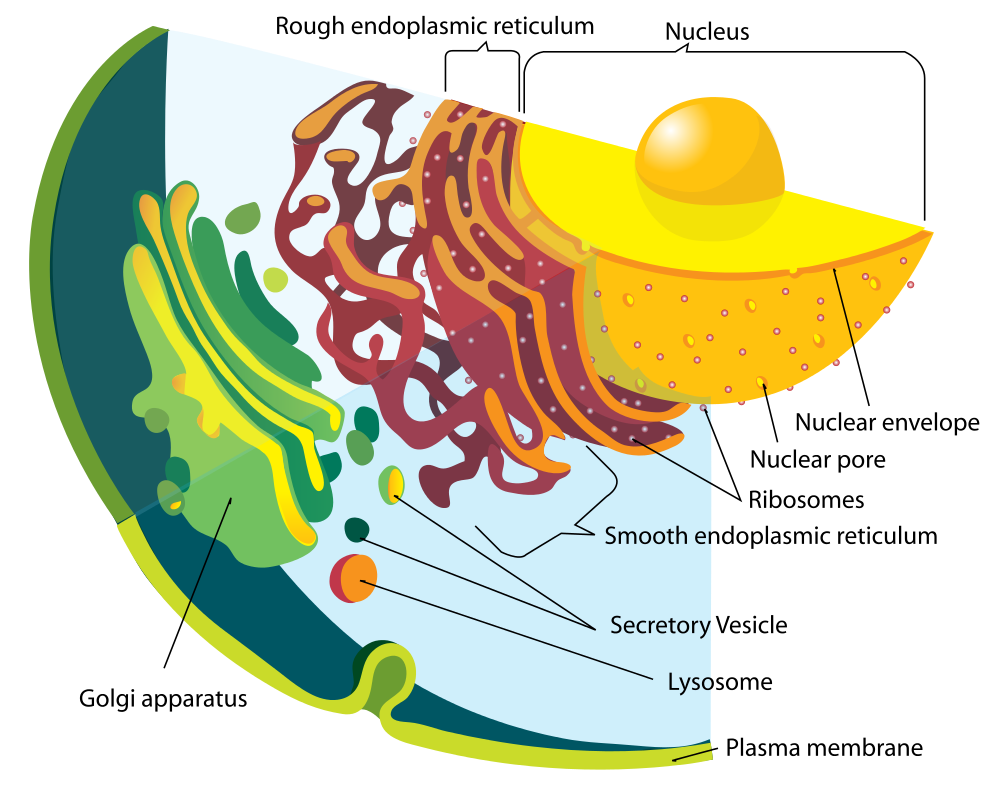


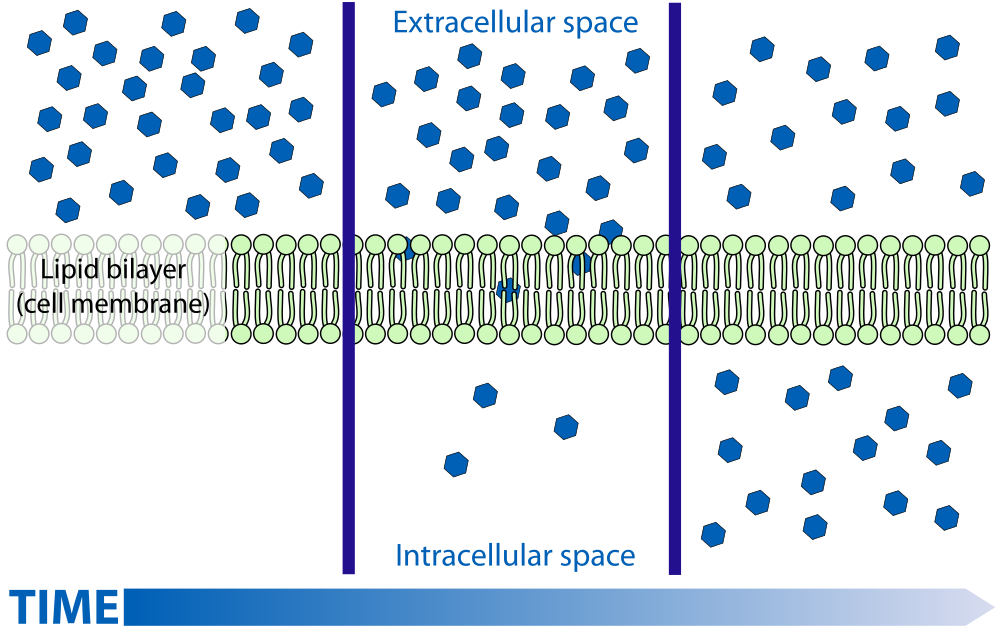
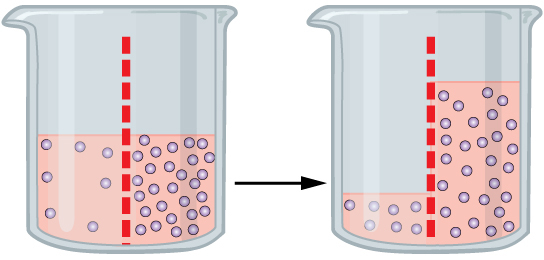
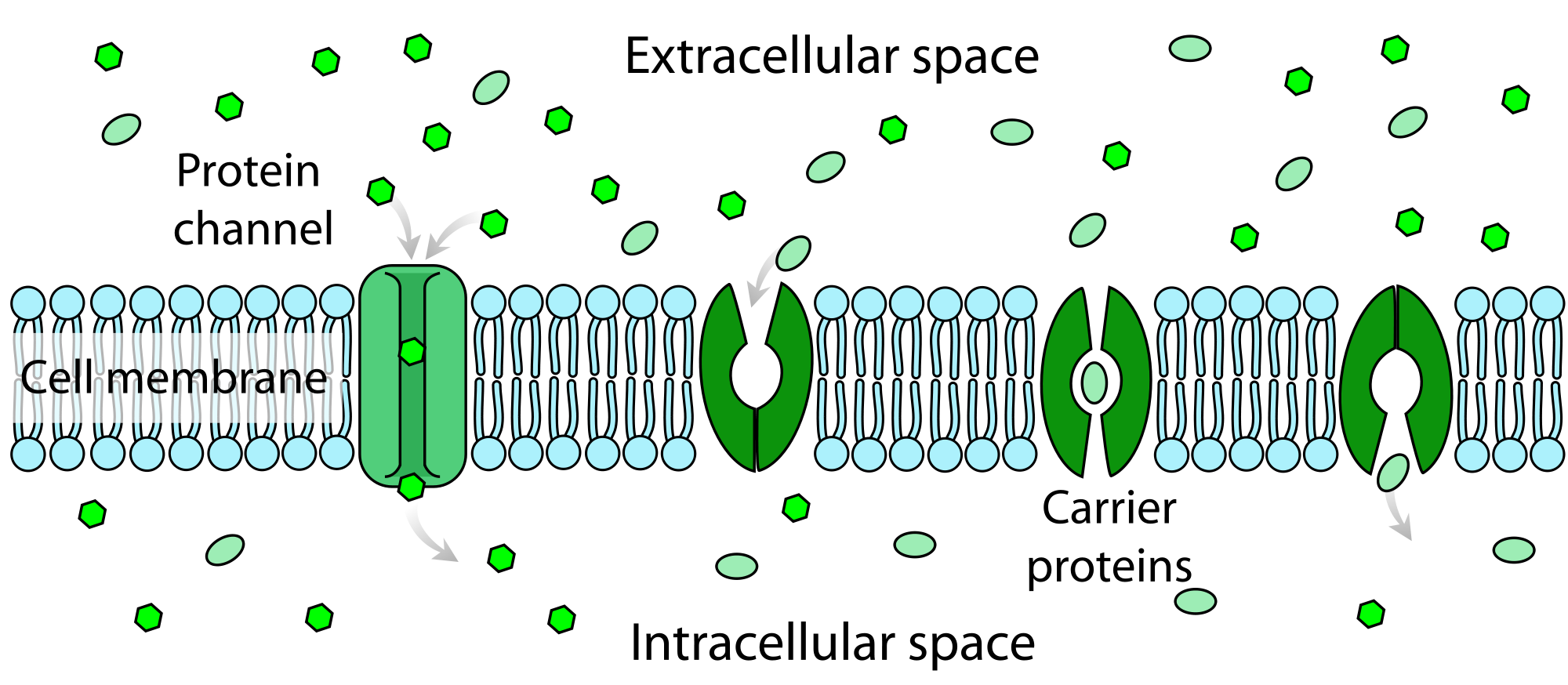



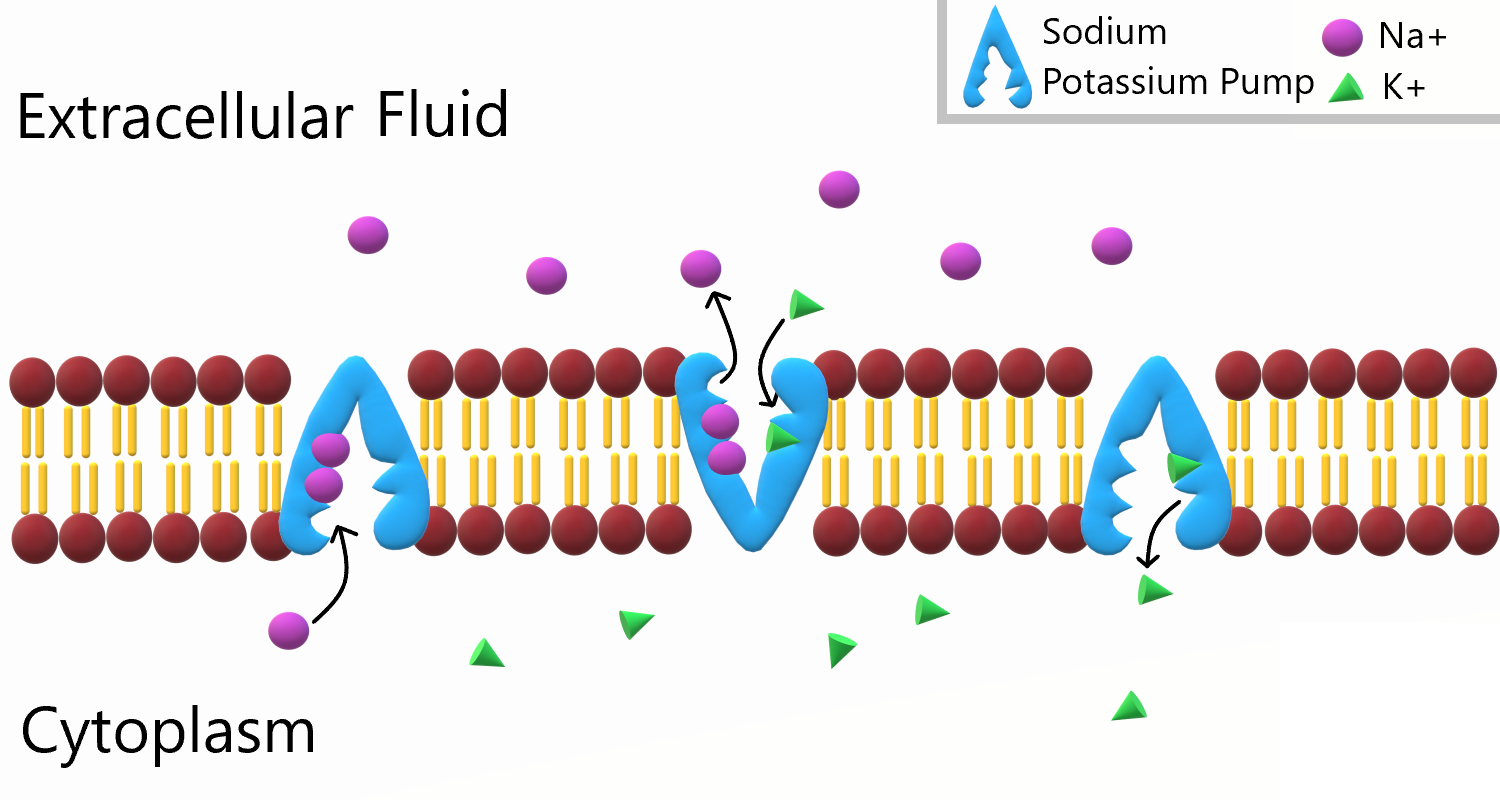
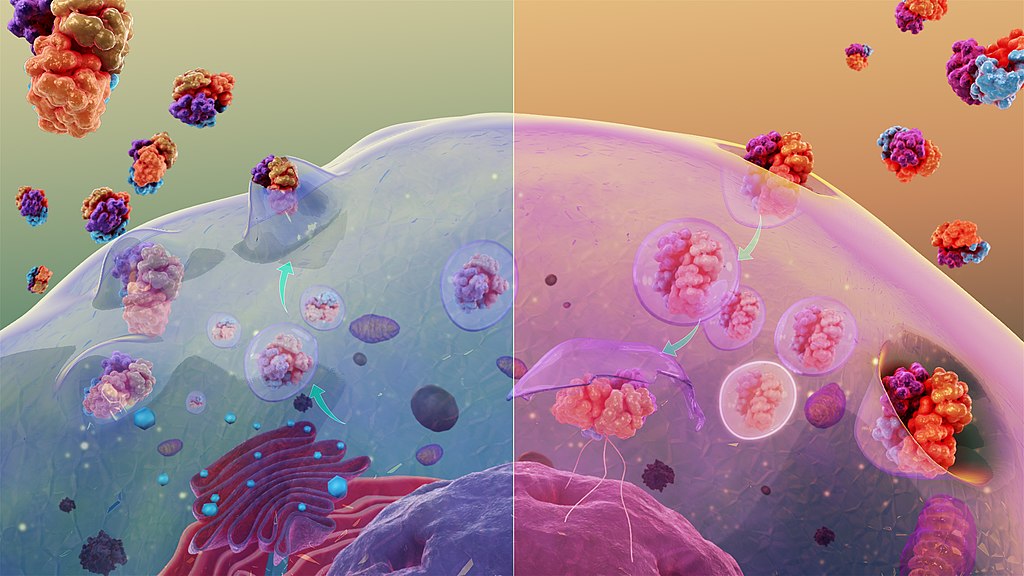






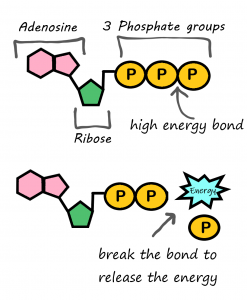
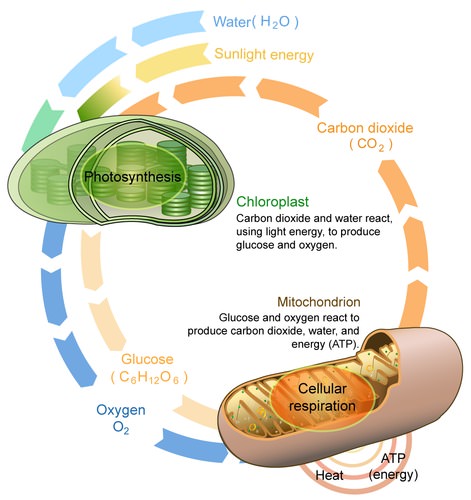


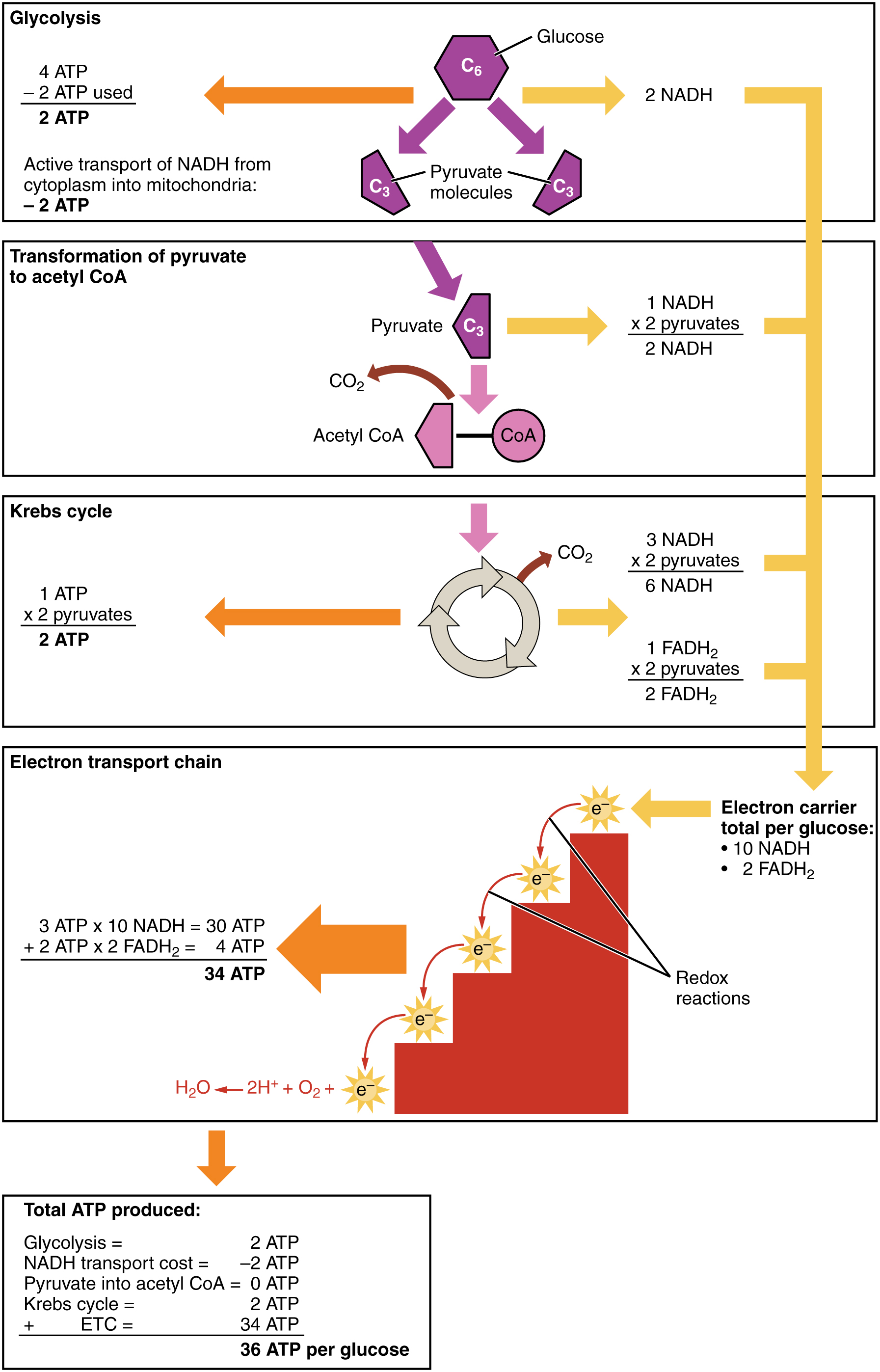
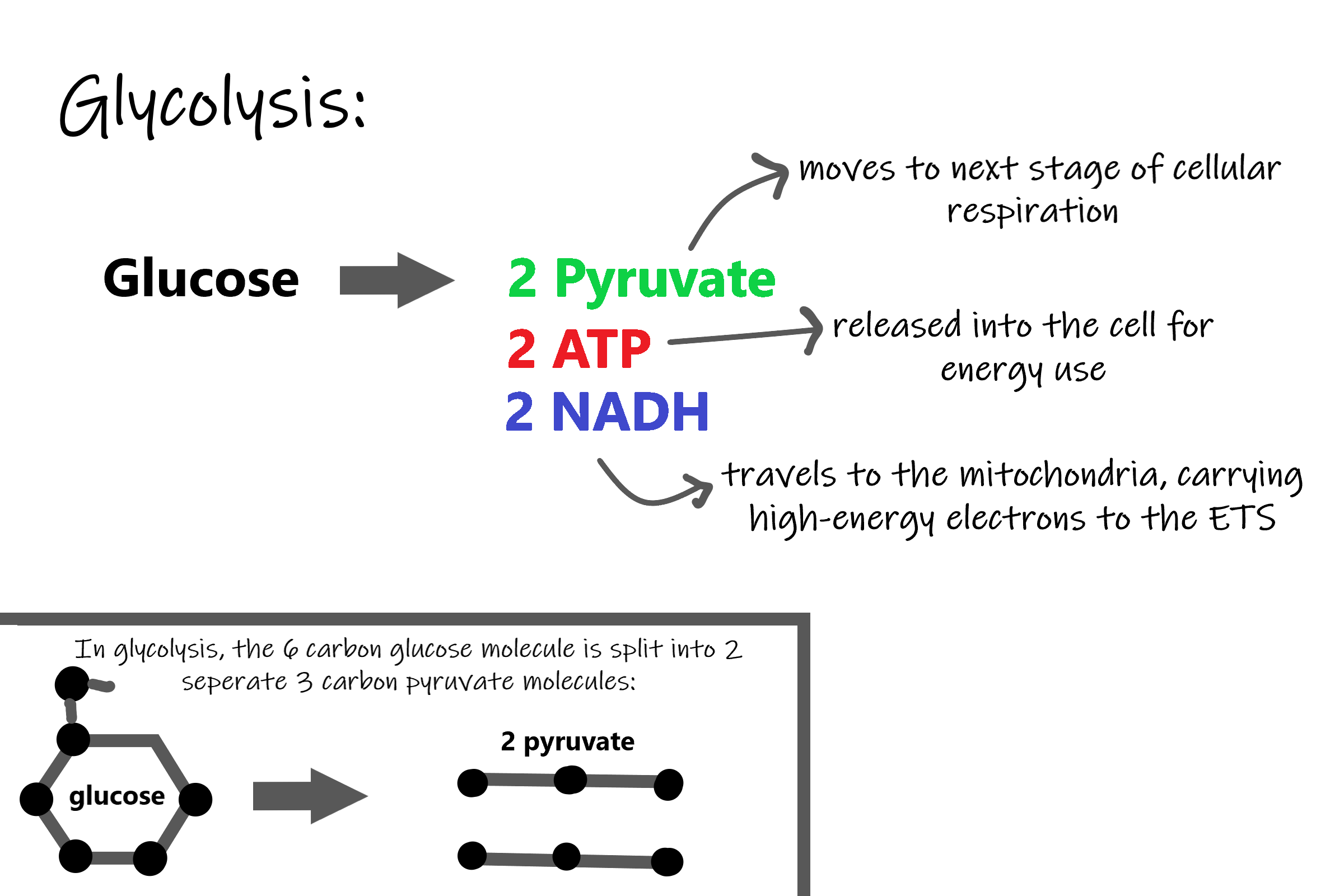
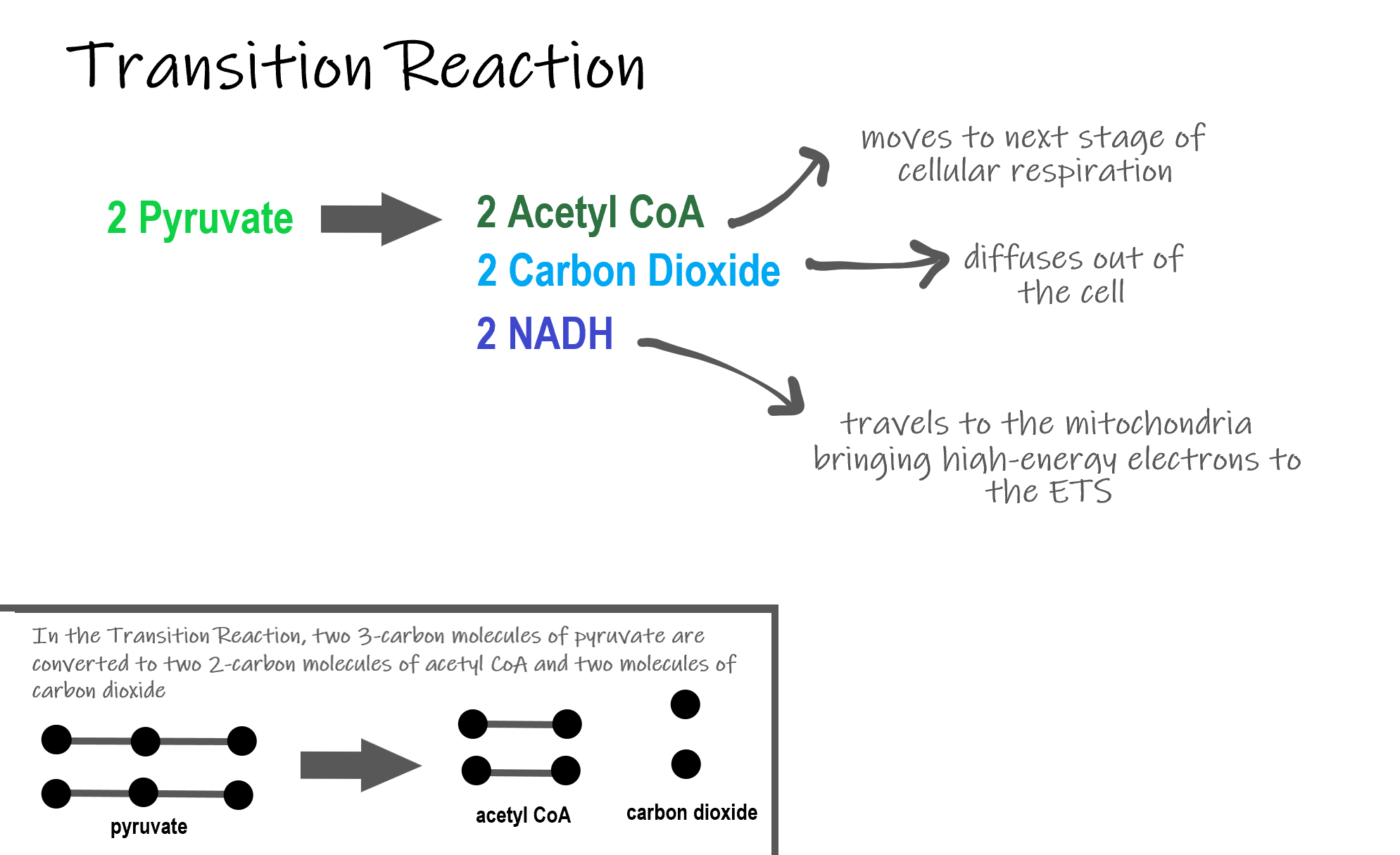
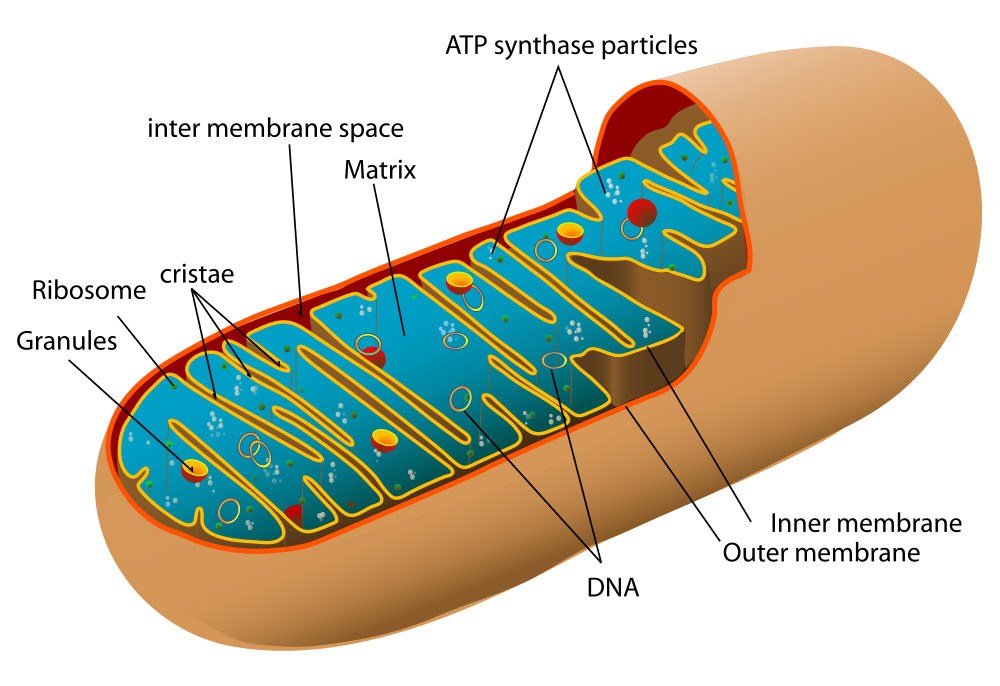
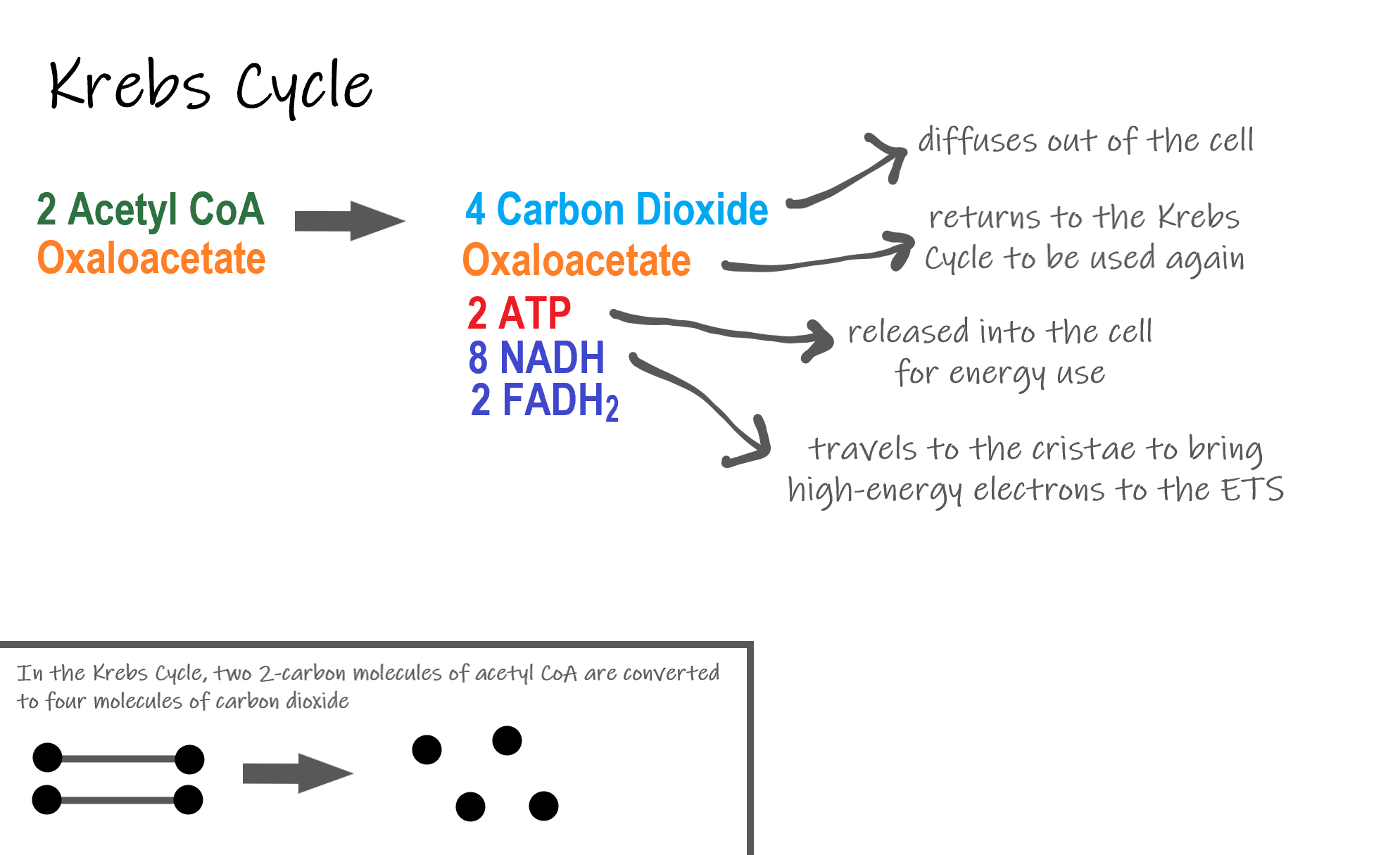
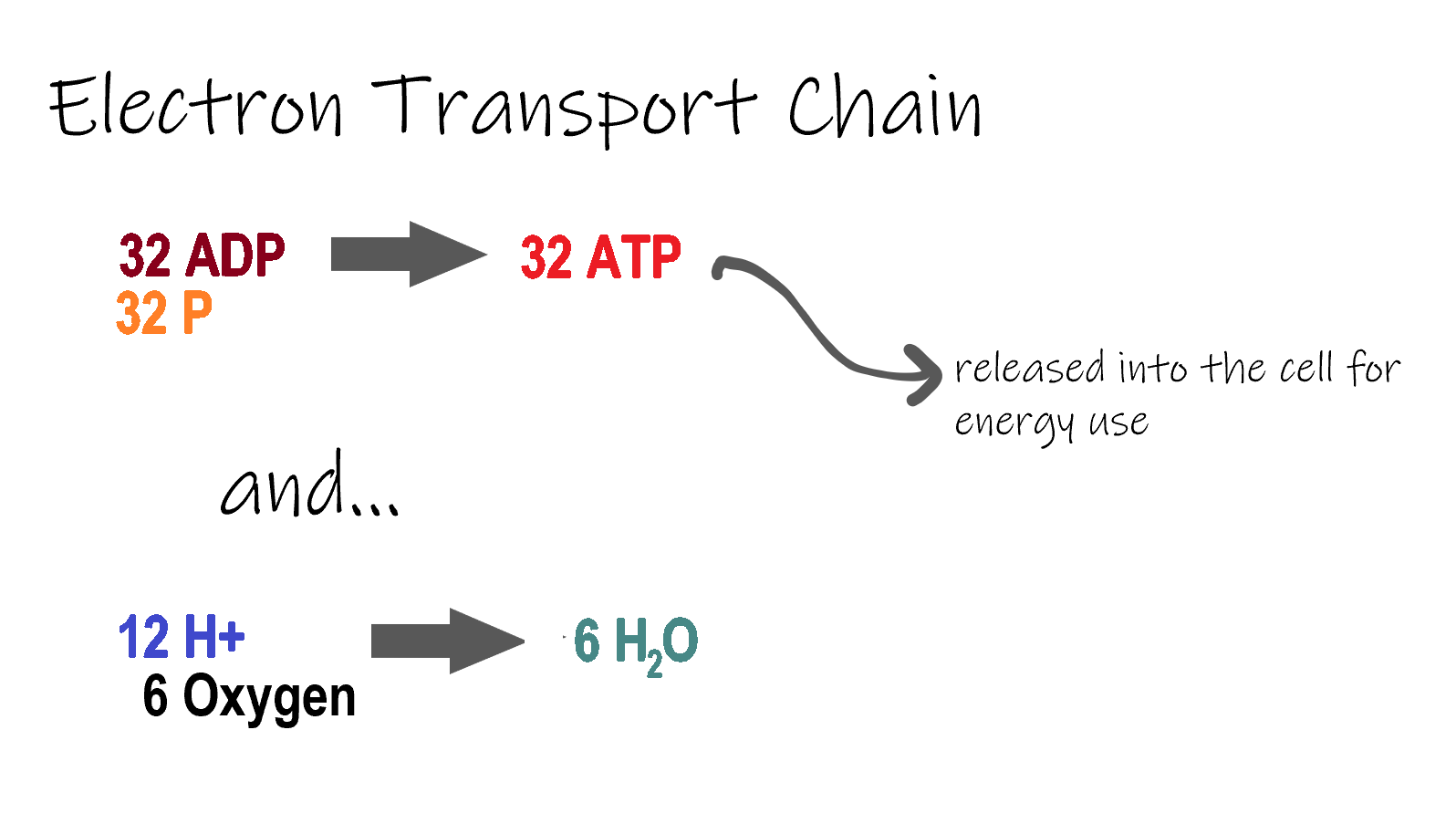










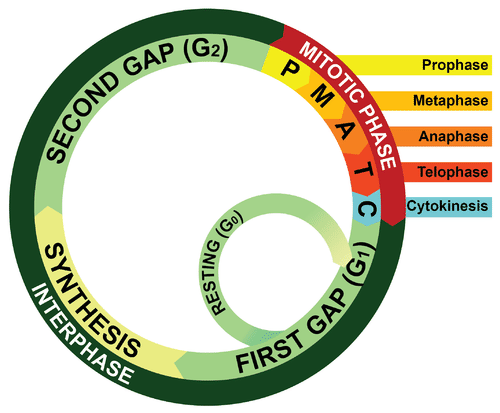
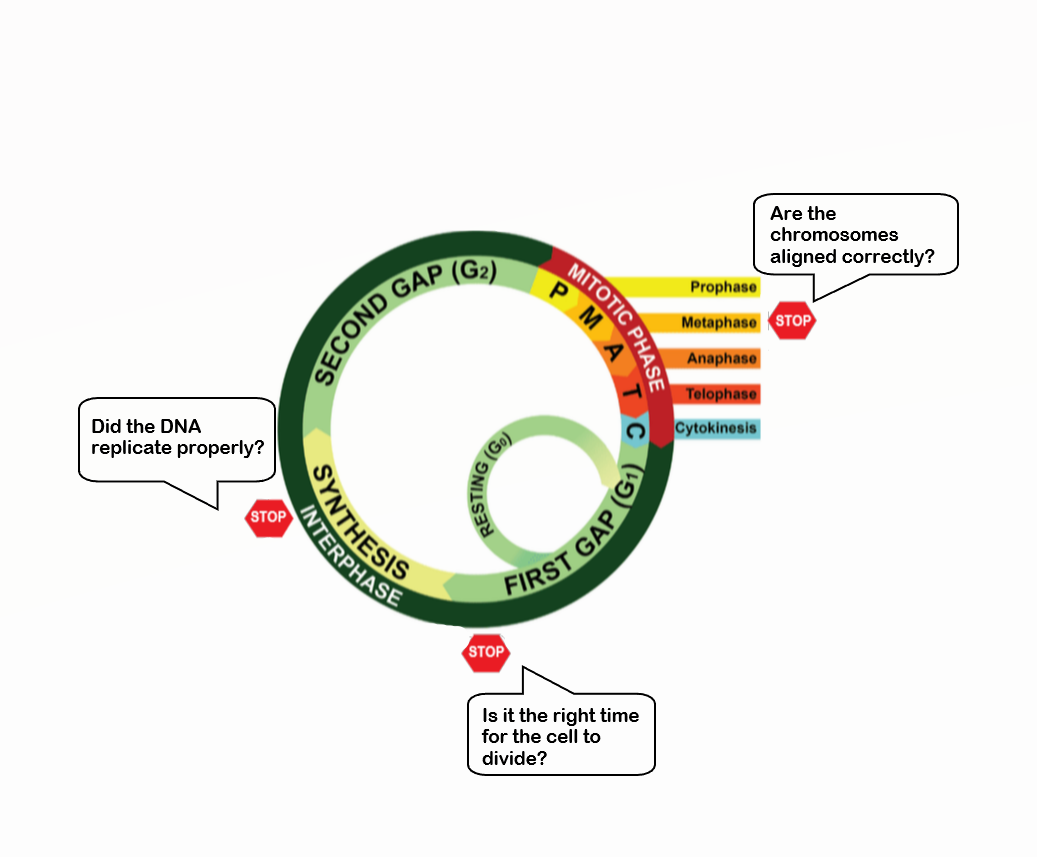



![The Cell Cycle (and cancer) [Updated] Thumbnail for the embedded element "The Cell Cycle (and cancer) [Updated]"](https://i.ytimg.com/vi/QVCjdNxJreE/hqdefault.jpg)